Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Triclosan (TCS), a kind of pharmaceuticals and personal care products (PPCPs), is widely used and has had a large production over years. It is an emerging pollutant in the water environment that has attracted global attention due to its toxic effects on organisms and aquatic ecosystems, and its concentrations in the water environment are expected to increase since the COVID-19 pandemic outbreak.
- triclosan
- water environment
- biodegradation
1. Introduction
Triclosan (5-chloro-2-(2’,4’-dichlorophenoxy) phenol, TCS) is an antibacterial agent that has been widely used in detergents, cosmetics, soaps, toothpaste and other pharmaceuticals and personal care products (PPCPs) since it was first marketed in 1957 [1][2]. TCS is a synthetic chlorinated aromatic compound. In the late 1930s and early 1940s, it was found that the substitution on aromatic rings of hydrogen atoms with chlorine can yield a kind of powerful biocides, including antimicrobials [3]. TCS is a kind of broad-spectrum antimicrobial, and it is widely found in surface water, soil and sediment [4][5]. Although the U.S. Food and Drug Administration issued the final rule on the safety and effectiveness of consumer preservatives in 2016, requiring the prohibition of the use of antimicrobials such as TCS in PPCPs such as soap, it still retains a large market demand [6].
TCS is a small molecular compound with controversial roles [7]. The bacteriostatic action of TCS preventing microbial growth is due to the inhibition of the enoyl–acyl carrier reductase, which is an enzyme involved in fatty acid synthesis [7][8]. However, the greatest concern for the potential health effects of TCS is related to its endocrine-disrupting activity [9]. TCS can also harm digestive, endocrine and reproductive development systems of aquatic organisms and human bodies [10][11][12][13].
In recent years, with the widespread use of TCS, the level of TCS in the water environment has been increasing, especially since the COVID-19 pandemic [14]. Wastewater discharge is the main source of TCS in the water environment. As one of the most concerned emerging pollutants in the world, TCS has also received extensive attention in its remediation research. In nutrient-poor aquatic or terrestrial ecosystems, TCS’s natural decay rate is very slow [15]. At present, some physical and chemical remediation methods such as photolysis, adsorption and oxidation have been applied for the removal of TCS in the environment. However, it is difficult to remove TCS in the actual treatment process due to its easy production of intermediates and high maintenance cost [15][16][17]. Studies have shown that TCS could be degraded by the ammonia-oxidizing bacteria (AOB) and heterotrophic bacteria in the water environment, and using microbial metabolism to degrade TCS in wastewater can lead to complete mineralization of TCS without producing toxic intermediate metabolites [1][18]. At present, with the great progress of isolation and culture technology, more and more TCS-degrading microorganisms and TCS-degrading microbial consortia have been obtained in the laboratory, and their degrading characteristics have been extensively studied.
2. Occurrence of TCS in the Water Environment
TCS in the water environment comes from the use of the hospital disinfectants, factory production of PPCPs wastewater and the use of PPCPs by humans. Wastewater containing TCS is discharged into Wastewater Treatment Plants (WWTPs), part of which is degraded by microorganisms, another is discharged into natural water with the effluent of WWTPs, and the other is discharged into the soil in the form of excess activated sludge. TCS in the soil enters the surface water and groundwater through the action of rainwater, and the surface water and groundwater are used as reference water sources [19]. The TCS enters the water purification plant, and the treated water is used as daily water [19]. This achieves the cycle of TCS in the water environment (Figure 1A).
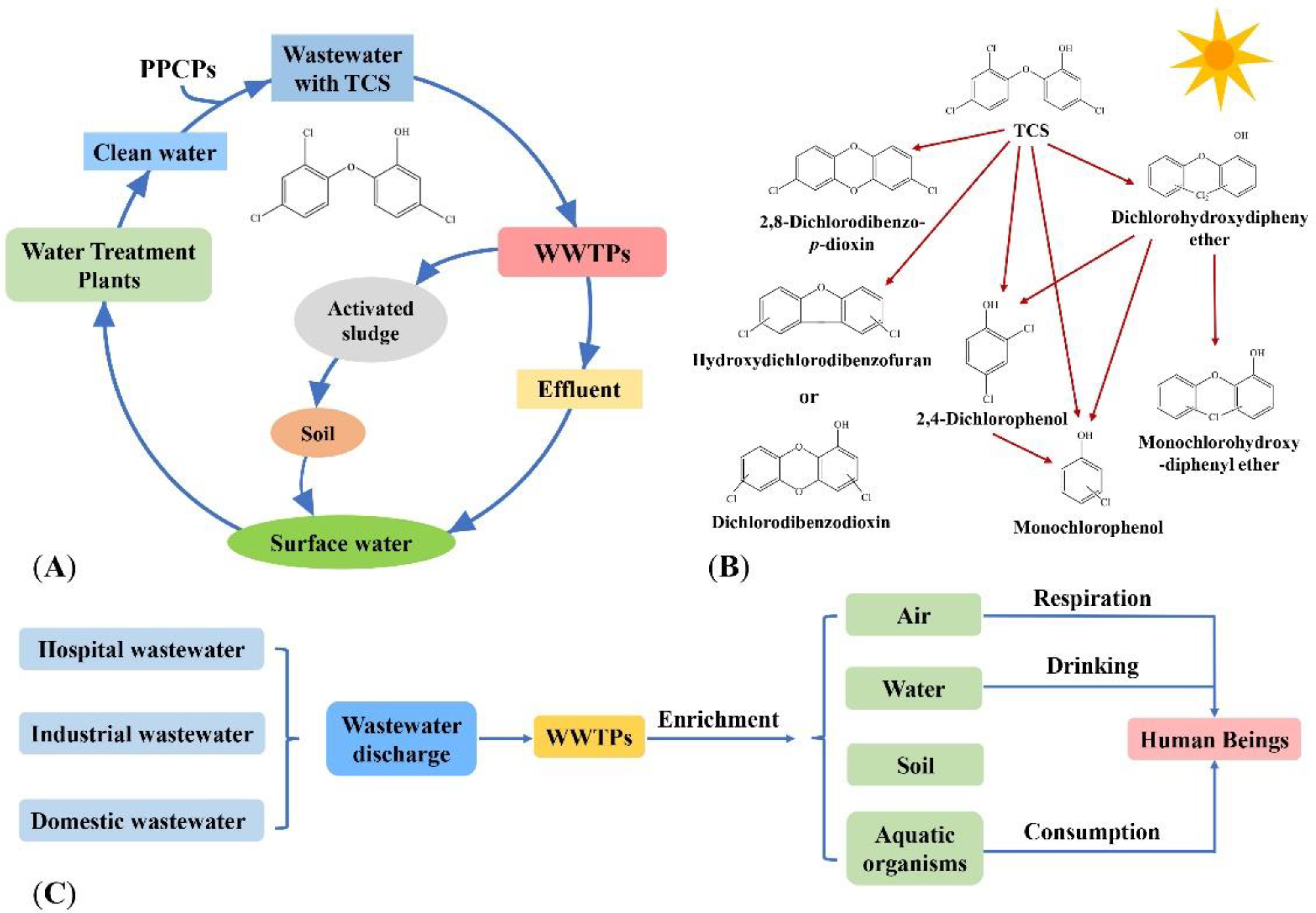
Figure 1. Occurrence of TCS in the water environment. (A) The circulation of TCS in the water environment. (B) Photodegradation products of TCS [20][21]. Reprinted with permission from Ref. [20]. 2022, Dar et al.; and Ref. [21]. 2008, Sanchez-Prado et al. (C) The fate of TCS in the water environment.
In the water environment, TCS will be transformed by photolysis into toxic substances such as 2,3-dichlorodibenzo-p-dioxin, dibenzodioxin and phenol intermediates, which are harmful to the aquatic organisms by bioaccumulation [22][23][24][25] (Figure 1B). Human beings ingest TCS through direct skin contact or consumption of TCS-containing products (e.g., fish that accumulate TCS), breathing TCS-containing air, and drinking TCS-containing water (Figure 1C).
Globally, it is estimated that 1500 tons of TCS produced annually, with approximately 132 million liters of TCS-containing products used in the U.S. alone [26]. The TCS productivity was significantly increased due to the high demand for disinfection since the outbreak of COVID-19 pandemic [27][28][29]. About 80% of TCS comes from cosmetics, household cleaning products and various PPCPs [20][30]. Domestic wastewater generated by humans through the use of TCS-containing PPCPs is considered to be a major source of TCS in wastewater [1][31].
After the wastewater-containing TCS is treated by WWTPs, part of TCS is adsorbed by the sludge, and the other part is degraded by microorganisms. The concentrations of TCS in the influent, effluent and sludge of some WWTPs are shown in Table 1.
Table 1. The concentrations of TCS in WWTPs.
| Country | Name of the WWTP | Processing Technology |
Concentration of TCS in Wastewater | Concentration of TCS in Sludge | Reference | ||
|---|---|---|---|---|---|---|---|
| Influent | Treated Effluent | Removal Rate/% | |||||
| China | Northern China WWTP | Anoxic-aerobic (A/O) | 295 ± 4.2 ng/L | 39 ± 2.7 ng/L | 86.77 | 1801 ng/g | [32] |
| Brazil | WWTP A | Activated sludge (AS) | 1.30 ± 0.22 μg/L | 0.55 ± 0.02 μg/L | 57.69% | 0.94 μg/L | [33] |
| WWTP B | Upflow anaerobic sludge blanket | 1.26 ± 0.09 μg/L | 0.78 ± 0.05 μg/L | 38.10% | 2.79 μg/L | ||
| WWTP C | Waste stabilization pond | 1.42 ± 0.04 μg/L | 0.39 ± 0.02 μg/L | 72.54% | 0.53 μg/L | ||
| Chile | WWTP | AS and a pilot plant of horizontal subsurface flow | 0.20 ± 0.06 μg/L | 0.02 ± 0.01 μg/L | 90.00% | 0.01 ± 0.01 μg/L | [34] |
| India | WWTP 1 | AS | N.A. 1 | N.A. 1 | 39–62% | N.A. 1 | [35] |
| WWTP 2 | 45–55% | ||||||
| China | WWTP#1 | A/O | 59–1100 ng/L Mean 274 ng/L |
13–110 ng/L Mean 83 ng/L |
69.71% | N.A. 1 | [36] |
| WWTP#2 | Hydrolytic acidification and cyclic activated sludge technology | 230–2900 ng/L Mean 389 ng/L |
9–180 ng/L Mean 17 ng/L |
95.63% | |||
1 ‘N.A.’ means that the relevant data are not available.
The way TCS enters the natural aquatic environment is mainly through wastewater discharge, so it is necessary to study the removal of TCS in WWTPs. As shown in Table 1, the presence of TCS was detected in both the influent and effluent of WWTPs. TCS was also detected in the sludge (Table 1). Heidler et al. [37] studied the removal effect of TCS by traditional WWTPs using a mass balance approach in conjunction with isotope dilution liquid chromatography electrospray ionization mass spectrometry for accurate quantification. The results showed that the fate of TCS during AS sewage treatment is mainly partitioned into three parts [37]. First, about half of the TCS was adsorbed and accumulated in AS (50 ± 19%); second, about 48 ± 19% of the TCS was actually transformed, lost or unaccounted for during treatment; third, an additional 2 ± 1% of the TCS was discharged into local surface water with the effluent [37]. Thus, it is difficult to achieve the complete removal of TCS by WWTPs.
TCS enters the environment through the effluent of WWTPs. It has been frequently detected in rivers, oceans and other surface water, although TCS has good lipophilic properties and is easily absorbed into sediment and sewage sludge (Table 2). Previous investigations have shown that TCS has a high detection rate in the environment [38]. A large survey conducted by the Joint Research Centre in 10 countries at 686 sampling sites found that TCS was detected in over 40% inland river samples [39]. In the US, the TCS detection rate in 139 rivers was 57.6% [40]. In China, the detection rate of TCS in the Yellow River, Yangtze River, Pearl River, Bohai Sea and East China Sea is more than 90% [39][40][41][42][43][44][45].
Table 2. The concentrations of TCS in different water environment.
| Environment | Method | TCS Concentration | Country | Year of the TCS Determination |
Reference |
|---|---|---|---|---|---|
| River water | Liquid chromatography-tandem mass spectrometry (LC-MS/MS) | N.D. 1 −62.124 µg/L | India | 2019–2020 | [46] |
| River water | LC-MS/MS | N.D. 1 −135 ng/L Mean 25.4 ng/L |
China | 2018, 2019 and 2021 | [43] |
| River water | Gas chromatography-mass spectrometer (GC-MS) | 0.06–500 ng/L Mean 176.2 ng/L |
Morocco | 2019 | [47] |
| River water | LC-MS/MS | Up to 74.3 µg/L | India | / 2 | [48] |
| River water | High-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) | N.D. 1 −1761 ng/L Mean 942 ng/L in monsoon season |
India | 2018–2019 | [49] |
| River water | Literature data collection | N.D. 1 −293.64 ng/L | China | 2010–2019 | [50] |
| River water | LC-MS/MS | 0.69–17.5 ng/L | China | 2019 | [42] |
| River water | LC-MS/MS | 5.1–874 ng/L Mean 0.06 nM |
Canada | 2012–2013 | [51] |
| River water | HPLC-MS/MS | N.D. −65.6 ng/L Mean 0.02 nM |
China | 2015 | [52] |
| River water | LC-LC-MS/MS | N.D. 1 −0.77 nM | Spain | 2012 | [53] |
| River water | High performance liquid chromatography with photo diode array detection | N.D. 1 −3.87 nM | South Africa | / 2 | [54] |
| River water | HPLC-MS/MS | 0.349 ± 0.032 nM | UK | / 2 | [55] |
| River water | GC-MS | 0.01–0.207 nM | Denmark | 2010 | [56] |
| Sea water | LC-MS/MS | N.D. 1 −58.3 ng/L Mean 22.3 ng/L |
China | 2018, 2019 and 2021 | [43] |
| Sea water | Ultra-performance liquid chromatography coupled to a triple quadrupole mass spectrometry | N.D. 1 −8.7 ng/L Mean 4.2 ng/L |
China | 2019 | [44] |
| Underground water | LC-MS/MS | 0.5–13.1 ng/L Mean 2.9 µg/L |
Poland | 2019 | [57] |
| Drinking water | LC-MS | Up to 9.74 ng/L | Malaysia | 2018 | [58] |
| Drinking water | GC-MS | 0.6–9.7 ng/L | China | / 2 | [59] |
1 ‘N.D.’ means that the relevant data are not detected. 2 ‘/’ means that the relevant information is not mentioned.
Currently, TCS has also been found to accumulate in algae, fishes, birds and other organisms [45][60][61]. Pashaei et al. [22] found that TCS has a concentration of 50–145 μg/kg in aquatic organisms such as Atlantic salmon, Atlantic sea wolf, rainbow trout, Atlantic cod, White vannamei prawn, Indian prawn and kiddi shrimp by HPLC-MS. TCS has also been detected in agricultural ecosystems with different trophic levels following the application of the WWTP biosolids to agricultural field, which means that TCS could be transferred through the terrestrial food web [25][62][63]. TCS was detected in human urine samples in China, the US, India, South Korea and other countries, with the highest average concentration of TCS in urine in China (100 ng/mL), while the lowest was in Vietnam (2.34 ng/mL) [64].
According to the published survey data, TCS is an emerging pollutant with a high frequency detected in the environment. Low concentrations of TCS can affect microorganisms and fish in aquatic environments and can be transmitted to humans through the food web, resulting in an adverse effect on human life and health. Therefore, it is necessary to study the degradation of TCS, especially its biodegradation.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091713
References
- Roh, H.; Subramanya, N.; Zhao, F.M.; Yu, C.P.; Sandt, J.; Chu, K.H. Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 2009, 77, 1084–1089.
- Macri, D. Worldwide Use of triclosan: Can dentistry do without this antimicrobial? Contemp. Clin. Dent. 2017, 8, 7–8.
- Halden, R.U. On the need and speed of regulating triclosan and triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611.
- Levy, C.W.; Roujeinikova, A.; Sedelnikova, S.; Baker, P.J.; Stuitje, A.R.; Slabas, A.R.; Rice, D.W.; Rafferty, J.B. Molecular basis of triclosan activity. Nature 1999, 398, 383–384.
- Fujimoto, M.; Carey, D.E.; McNamara, P.J. Metagenomics reveal triclosan-induced changes in the antibiotic resistome of anaerobic digesters. Environ. Pollut. 2018, 241, 1182–1190.
- Halden, R.U.; Paull, D.H. Co-occurrence of triclocarban and triclosan in US water resources. Environ. Sci. Technol. 2005, 39, 1420–1426.
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A small molecule with controversial roles. Antibiotics 2022, 11, 735.
- Ates, G.; Goldberg, J.; Currais, A.; Maher, P. CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biol. 2020, 36, 101648.
- Contardo-Jara, V.; Meinecke, S.; Feibicke, M.; Berghahn, R.; Schmidt, R.; Mohr, S. Fate, bioaccumulation and toxic effects of triclosan on a freshwater community–A mesocosm study. Environ. Adv. 2021, 5, 100100.
- Zhang, J.; Walker, M.E.; Sanidad, K.Z.; Zhang, H.N.; Liang, Y.S.; Zhao, E.M.; Chacon-Vargas, K.; Yeliseyev, V.; Parsonnet, J.; Haggerty, T.D.; et al. Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract. Nat. Commun. 2022, 13, 136.
- Yee, A.L.; Gilbert, J.A. Is triclosan harming your microbiome? Science 2016, 353, 348–349.
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.L.; Cheroni, C.; Borbely, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, eabe8244.
- Yuan, G.X.; Ma, Y.; Zeng, Y.X.; Pan, H.B.; Liu, P.Y.; Liu, Y.; Liu, G.H.; Cheng, J.Q.; Guo, Y.S. Associations between low-dose triclosan exposure and semen quality in a Chinese population. Environ. Pollut. 2022, 299, 118926.
- Lu, J.; Guo, J.H. Disinfection spreads antimicrobial resistance. Science 2021, 371, 474.
- Mulla, S.I.; Asefi, B.; Bharagava, R.N.; Saratale, G.D.; Li, J.W.; Huang, C.L.; Yu, C.P. Processes for the removal of triclosan in the environment and engineered systems: A review. Environ. Rev. 2020, 28, 55–66.
- Sola-Gutierrez, C.; Schroder, S.; San-Roman, M.F.; Ortiz, I. Critical review on the mechanistic photolytic and photocatalytic degradation of triclosan. J. Environ. Manag. 2020, 260, 110101.
- Quan, B.Y.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.C.; Xi, Y.N.; Xu, W.H.; Liu, Y.G.; Tang, Y.Q. Technology and principle of removing triclosan from aqueous media: A review. Chem. Eng. J. 2019, 378, 122185.
- Balakrishnan, P.; Mohan, S. Treatment of triclosan through enhanced microbial biodegradation. J. Hazard. Mater. 2021, 420, 126430.
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065.
- Dar, O.I.; Raouf, A.; Deng, P.; Sunil, S.; Megha, A.; Kaur, A.; Jia, A.Q.; Faggio, C. Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: A review. Environ. Technol. Innov. 2022, 25, 102122.
- Sanchez-Prado, L.; Barro, R.; Garcia-Jares, C.; Llompart, M.; Lores, M.; Petrakis, C.; Kalogerakis, N.; Mantzavinos, D.; Psillakis, E. Sonochemical degradation of triclosan in water and wastewater. Ultrason. Sonochemistry 2008, 15, 689–694.
- Pashaei, R.; Dzingeleviciene, R.; Abbasi, S.; Szultka-Mlynska, M.; Buszewski, B. Determination of 15 human pharmaceutical residues in fish and shrimp tissues by high-performance liquid chromatography-tandem mass spectrometry. Environ. Monit. Assess 2022, 194, 325.
- Roberts, J.; Price, O.R.; Bettles, N.; Rendal, C.; van Egmond, R. Accounting for dissociation and photolysis: A review of the algal toxicity of triclosan. Environ. Toxicol. Chem. 2014, 33, 2551–2559.
- Wu, J.L.; Ji, F.F.; Zhang, H.N.; Hu, C.Q.; Wong, M.H.; Hu, D.; Cai, Z.W. Formation of dioxins from triclosan with active chlorine: A potential risk assessment. J. Hazard. Mater. 2019, 367, 128–136.
- Yuan, X.; Hu, J.T.; Li, S.Y.; Yu, M.Z. Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Environ. Pollut. 2020, 266, 115340.
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive insight into triclosan—From widespread occurrence to health outcomes. Environ. Sci. Pollut. Res. 2021.
- Chu, W.H.; Fang, C.; Deng, Y.; Xu, Z.X. Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety. Environ. Sci. Technol. 2021, 55, 4084–4086.
- Wang, M.L.; Hu, B.Y.; Zhou, W.; Huang, K.; Fu, J.J.; Zhang, A.Q.; Jiang, G.B. Enhanced hand-to-mouth exposure from hand sanitizers during the COVID-19 pandemic: A case study of triclosan. Sci. Bullet 2022, 67, 995–998.
- Usman, M.; Farooq, M.; Hanna, K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020, 745, 141053.
- Bilal, M.; Barcelo, D.; Iqbal, H.M.N. Persistence, ecological risks, and oxidoreductases-assisted biocatalytic removal of triclosan from the aquatic environment. Sci. Total Environ. 2020, 735, 139194.
- Wang, Y.W.; Liang, W. Occurrence, toxicity, and removal methods of triclosan: A timely review. Curr. Pollut. Rep. 2021, 7, 31–39.
- Zhang, Z.F.; Wang, L.; Zhang, X.M.; Zhang, X.; Li, Y.F.; Nikolaev, A.; Li, W.L. Fate processes of parabens, triclocarban and triclosan during wastewater treatment: Assessment via field measurements and model simulations. Environ. Sci. Pollut. Res. 2021, 28, 50602–50610.
- Komolafe, O.; Mrozik, W.; Dolfing, J.; Acharya, K.; Vassalle, L.; Mota, C.R.; Davenport, R. Occurrence and removal of micropollutants in full-scale aerobic, anaerobic and facultative wastewater treatment plants in Brazil. J. Environ. Manag. 2021, 287, 112286.
- Contreras, C.R.; Lopez, D.; Leiva, A.M.; Dominguez, C.; Bayona, J.M.; Vidal, G. Removal of organic micropollutants in wastewater treated by activated sludge and constructed wetlands: A comparative study. Water 2020, 11, 2515.
- Mohan, S.; Balakrishnan, P. Triclosan in treated wastewater from a city wastewater treatment plant and its environmental risk assessment. Water Air Soil Pollut. 2019, 230, 69.
- Li, W.L.; Zhang, Z.F.; Ma, W.L.; Liu, L.Y.; Song, W.W.; Li, Y.F. An evaluation on the intra-day dynamics, seasonal variations and removal of selected pharmaceuticals and personal care products from urban wastewater treatment plants. Sci. Total Environ. 2018, 640, 1139–1147.
- Heidler, J.; Halden, R.U. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 66, 362–369.
- Zheng, G.D.; Yu, B.; Wang, Y.W.; Ma, C.; Chen, T.B. Removal of triclosan during wastewater treatment process and sewage sludge composting-A case study in the middle reaches of the Yellow River. Environ. Int. 2020, 134, 105300.
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51.
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211.
- Chen, Z.F.; Ying, G.G.; Liu, Y.S.; Zhang, Q.Q.; Zhao, J.L.; Liu, S.S.; Chen, J.; Peng, F.J.; Lai, H.J.; Pan, C.G. Triclosan as a surrogate for household biocides: An investigation into biocides in aquatic environments of a highly urbanized region. Water Res. 2014, 58, 269–279.
- Chen, P.; Zhong, Y.; Chen, K.C.; Guo, C.S.; Gong, J.; Wang, D.D.; Yang, Y.; Ma, S.T.; Yu, Y.X. The impact of discharge reduction activities on the occurrence of contaminants of emerging concern in surface water from the Pearl River. Environ. Sci. Pollut. Res. 2020, 27, 30378–30389.
- Lu, S.; Wang, B.D.; Xin, M.; Wang, J.; Gu, X.; Lian, M.S.; Li, Y.; Lin, C.Y.; Ouyang, W.; Liu, X.T.; et al. Insights into the spatiotemporal occurrence and mixture risk assessment of household and personal care products in the waters from rivers to Laizhou Bay, southern Bohai Sea. Sci. Total Environ. 2022, 810, 152290.
- Xie, J.H.; Zhao, N.; Zhang, Y.Y.; Hu, H.M.; Zhao, M.R.; Jin, H.B. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2022, 287, 132218.
- Liu, X.Y.; Tu, M.C.; Wang, S.P.; Wang, Y.Z.; Wang, J.; Hou, Y.; Zheng, X.; Yan, Z.G. Research on freshwater water quality criteria, sediment quality criteria and ecological risk assessment of triclosan in China. Sci. Total Environ. 2022, 816, 151616.
- Saha, S.; Narayanan, N.; Singh, N.; Gupta, S. Occurrence of endocrine disrupting chemicals (EDCs) in river water, ground water and agricultural soils of India. Int. J. Environ. Sci. Technol. 2022.
- Chafi, S.; Azzouz, A.; Ballesteros, E. Occurrence and distribution of endocrine disrupting chemicals and pharmaceuticals in the river Bouregreg (Rabat, Morocco). Chemosphere 2021, 287, 132202.
- Kachhawaha, A.S.; Nagarnaik, P.M.; Labhasetwar, P.K.; Banerjee, K. Pharmaceuticals and personal care products in aqueous urban environment of western India. Water Environ. J. 2021, 35, 1302–1312.
- Gopal, C.M.; Bhat, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Udayashankar, H.N.; Vasantharaju, S.G.; Praveenkumarreddy, Y.; Shailesh; et al. Seasonal occurrence and risk assessment of pharmaceutical and personal care products in Bengaluru rivers and lakes, India. J. Environ. Chem. Eng. 2021, 9, 105610.
- Xiang, Y.; Wu, H.H.; Li, L.; Ren, M.; Qie, H.T.; Lin, A.J. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044.
- Lalonde, B.; Garron, C.; Dove, A.; Struger, J.; Farmer, K.; Sekela, M.; Gledhill, M.; Backus, S. Investigation of spatial distributions and temporal trends of triclosan in Canadian surface waters. Arch. Environ. Contam. Toxicol. 2019, 76, 231–245.
- Ma, X.Q.; Wan, Y.J.; Wu, M.Y.; Xu, Y.; Xu, Q.; He, Z.Y.; Xia, W. Occurrence of benzophenones, parabens and triclosan in the Yangtze River of China, and the implications for human exposure. Chemosphere 2018, 213, 517–525.
- Esteban, S.; Gorga, M.; Petrovic, M.; Gonzalez-Alonso, S.; Barcelo, D.; Valcarcel, Y. Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 2014, 466–467, 939–951.
- Madikizela, L.M.; Muthwa, S.F.; Chimuka, L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S. Afr. J. Chem. 2014, 67, 143–150.
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78.
- Matamoros, V.; Arias, C.A.; Nguyen, L.X.; Salvado, V.; Brix, H. Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 2012, 88, 1083–1089.
- Rusiniak, P.; Kmiecik, E.; Wator, K.; Duda, R.; Bugno, R. Pharmaceuticals and personal care products in the urban groundwater—Preliminary monitoring (case study: Krakow, Southern Poland). Urban Water J. 2021, 18, 364–374.
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence of multiclass endocrine disrupting compounds in a drinking water supply system and associated risks. Sci. Rep. 2020, 10, 17755.
- Li, X.; Ying, G.G.; Su, H.C.; Yang, X.B.; Wang, L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36, 557–562.
- Tang, N.; Fan, P.P.; Yu, X.G.; Ma, R.; Tao, Y.X.; Wang, W.Y.; Ouyang, F.X. Effects of long-term triclosan exposure on microbiota in zebrafish. Front. Microbiol. 2021, 12, 604313.
- Rüdela, H.; Böhmera, W.; Müller, M. Retrospective study of triclosan and methyl-triclosan residues in fish and suspended particulate matter: Results from the German Environmental Specimen Bank. Chemosphere 2013, 91, 1517–1524.
- Yun, H.; Liang, B.; Kong, D.; Li, X.K.; Wang, A.J. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944.
- Sherburne, J.J.; Anaya, A.M.; Fernie, K.J.; Forbey, J.S.; Furlong, E.T.; Kolpin, D.W.; Dufty, A.M.; Kinney, C.A. Occurrence of triclocarban and triclosan in an agro-ecosystem following application of biosolids. Environ. Sci. Technol. 2016, 50, 13206–13214.
- Iyer, A.P.; Xue, J.C.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96.
This entry is offline, you can click here to edit this entry!
