Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
|
Water Resources
Triclosan (TCS), a kind of pharmaceuticals and personal care products (PPCPs), is widely used and has had a large production over years. It is an emerging pollutant in the water environment that has attracted global attention due to its toxic effects on organisms and aquatic ecosystems, and its concentrations in the water environment are expected to increase since the COVID-19 pandemic outbreak.
- triclosan
- water environment
- biodegradation
1. Introduction
Triclosan (5-chloro-2-(2’,4’-dichlorophenoxy) phenol, TCS) is an antibacterial agent that has been widely used in detergents, cosmetics, soaps, toothpaste and other pharmaceuticals and personal care products (PPCPs) since it was first marketed in 1957 [1,2]. TCS is a synthetic chlorinated aromatic compound, its basic physical and chemical properties are shown in Table S1. In the late 1930s and early 1940s, it was found that the substitution on aromatic rings of hydrogen atoms with chlorine can yield a kind of powerful biocides, including antimicrobials [3]. TCS is a kind of broad-spectrum antimicrobial, and it is widely found in surface water, soil and sediment [4,5]. Although the U.S. Food and Drug Administration issued the final rule on the safety and effectiveness of consumer preservatives in 2016, requiring the prohibition of the use of antimicrobials such as TCS in PPCPs such as soap, it still retains a large market demand [6].
TCS is a small molecular compound with controversial roles [7]. The bacteriostatic action of TCS preventing microbial growth is due to the inhibition of the enoyl–acyl carrier reductase, which is an enzyme involved in fatty acid synthesis [7,8]. However, the greatest concern for the potential health effects of TCS is related to its endocrine-disrupting activity [9]. TCS can also harm digestive, endocrine and reproductive development systems of aquatic organisms and human bodies [10,11,12,13].
In recent years, with the widespread use of TCS, the level of TCS in the water environment has been increasing, especially since the COVID-19 pandemic [14]. Wastewater discharge is the main source of TCS in the water environment. As one of the most concerned emerging pollutants in the world, TCS has also received extensive attention in its remediation research. In nutrient-poor aquatic or terrestrial ecosystems, TCS’s natural decay rate is very slow [15]. At present, some physical and chemical remediation methods such as photolysis, adsorption and oxidation have been applied for the removal of TCS in the environment. However, it is difficult to remove TCS in the actual treatment process due to its easy production of intermediates and high maintenance cost [15,16,17]. Studies have shown that TCS could be degraded by the ammonia-oxidizing bacteria (AOB) and heterotrophic bacteria in the water environment, and using microbial metabolism to degrade TCS in wastewater can lead to complete mineralization of TCS without producing toxic intermediate metabolites [1,18]. At present, with the great progress of isolation and culture technology, more and more TCS-degrading microorganisms and TCS-degrading microbial consortia have been obtained in the laboratory, and their degrading characteristics have been extensively studied.
2. Occurrence of TCS in the Water Environment
TCS in the water environment comes from the use of the hospital disinfectants, factory production of PPCPs wastewater and the use of PPCPs by humans. Wastewater containing TCS is discharged into Wastewater Treatment Plants (WWTPs), part of which is degraded by microorganisms, another is discharged into natural water with the effluent of WWTPs, and the other is discharged into the soil in the form of excess activated sludge. TCS in the soil enters the surface water and groundwater through the action of rainwater, and the surface water and groundwater are used as reference water sources [27]. The TCS enters the water purification plant, and the treated water is used as daily water [27]. This achieves the cycle of TCS in the water environment (Figure 1A).
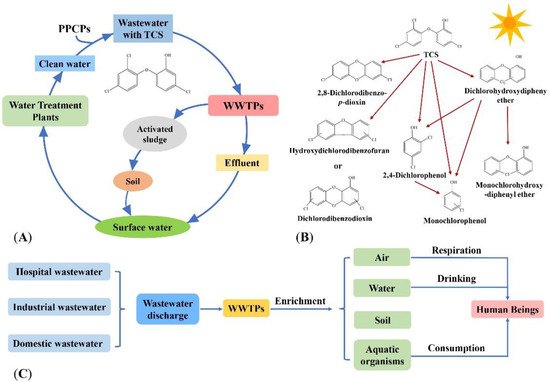
Figure 1. Occurrence of TCS in the water environment. (A) The circulation of TCS in the water environment. (B) Photodegradation products of TCS [32,33]. Reprinted with permission from Ref. [32]. 2022, Dar et al.; and Ref. [33]. 2008, Sanchez-Prado et al. (C) The fate of TCS in the water environment.
In the water environment, TCS will be transformed by photolysis into toxic substances such as 2,3-dichlorodibenzo-p-dioxin, dibenzodioxin and phenol intermediates, which are harmful to the aquatic organisms by bioaccumulation [28,29,30,31] (Figure 1B). Human beings ingest TCS through direct skin contact or consumption of TCS-containing products (e.g., fish that accumulate TCS), breathing TCS-containing air, and drinking TCS-containing water (Figure 1C).
Globally, it is estimated that 1500 tons of TCS produced annually, with approximately 132 million liters of TCS-containing products used in the U.S. alone [19]. The TCS productivity was significantly increased due to the high demand for disinfection since the outbreak of COVID-19 pandemic [25,34,35]. About 80% of TCS comes from cosmetics, household cleaning products and various PPCPs [32,36]. Domestic wastewater generated by humans through the use of TCS-containing PPCPs is considered to be a major source of TCS in wastewater [1,37].
After the wastewater-containing TCS is treated by WWTPs, part of TCS is adsorbed by the sludge, and the other part is degraded by microorganisms. The concentrations of TCS in the influent, effluent and sludge of some WWTPs are shown in Table 1.
Table 1. The concentrations of TCS in WWTPs.
| Country | Name of the WWTP | Processing Technology |
Concentration of TCS in Wastewater | Concentration of TCS in Sludge | Reference | ||
|---|---|---|---|---|---|---|---|
| Influent | Treated Effluent | Removal Rate/% | |||||
| China | Northern China WWTP | Anoxic-aerobic (A/O) | 295 ± 4.2 ng/L | 39 ± 2.7 ng/L | 86.77 | 1801 ng/g | [38] |
| Brazil | WWTP A | Activated sludge (AS) | 1.30 ± 0.22 μg/L | 0.55 ± 0.02 μg/L | 57.69% | 0.94 μg/L | [39] |
| WWTP B | Upflow anaerobic sludge blanket | 1.26 ± 0.09 μg/L | 0.78 ± 0.05 μg/L | 38.10% | 2.79 μg/L | ||
| WWTP C | Waste stabilization pond | 1.42 ± 0.04 μg/L | 0.39 ± 0.02 μg/L | 72.54% | 0.53 μg/L | ||
| Chile | WWTP | AS and a pilot plant of horizontal subsurface flow | 0.20 ± 0.06 μg/L | 0.02 ± 0.01 μg/L | 90.00% | 0.01 ± 0.01 μg/L | [40] |
| India | WWTP 1 | AS | N.A. 1 | N.A. 1 | 39–62% | N.A. 1 | [41] |
| WWTP 2 | 45–55% | ||||||
| China | WWTP#1 | A/O | 59–1100 ng/L Mean 274 ng/L |
13–110 ng/L Mean 83 ng/L |
69.71% | N.A. 1 | [42] |
| WWTP#2 | Hydrolytic acidification and cyclic activated sludge technology | 230–2900 ng/L Mean 389 ng/L |
9–180 ng/L Mean 17 ng/L |
95.63% | |||
1 ‘N.A.’ means that the relevant data are not available.
The way TCS enters the natural aquatic environment is mainly through wastewater discharge, so it is necessary to study the removal of TCS in WWTPs. As shown in Table 1, the presence of TCS was detected in both the influent and effluent of WWTPs. TCS was also detected in the sludge (Table 1). Heidler et al. [43] studied the removal effect of TCS by traditional WWTPs using a mass balance approach in conjunction with isotope dilution liquid chromatography electrospray ionization mass spectrometry for accurate quantification. The results showed that the fate of TCS during AS sewage treatment is mainly partitioned into three parts [43]. First, about half of the TCS was adsorbed and accumulated in AS (50 ± 19%); second, about 48 ± 19% of the TCS was actually transformed, lost or unaccounted for during treatment; third, an additional 2 ± 1% of the TCS was discharged into local surface water with the effluent [43]. Thus, it is difficult to achieve the complete removal of TCS by WWTPs.
TCS enters the environment through the effluent of WWTPs. It has been frequently detected in rivers, oceans and other surface water, although TCS has good lipophilic properties and is easily absorbed into sediment and sewage sludge (Table 2). Previous investigations have shown that TCS has a high detection rate in the environment [44]. A large survey conducted by the Joint Research Centre in 10 countries at 686 sampling sites found that TCS was detected in over 40% inland river samples [45]. In the US, the TCS detection rate in 139 rivers was 57.6% [46]. In China, the detection rate of TCS in the Yellow River, Yangtze River, Pearl River, Bohai Sea and East China Sea is more than 90% [45,46,47,48,49,50,51].
Table 2. The concentrations of TCS in different water environment.
| Environment | Method | TCS Concentration | Country | Year of the TCS Determination |
Reference |
|---|---|---|---|---|---|
| River water | Liquid chromatography-tandem mass spectrometry (LC-MS/MS) | N.D. 1 −62.124 µg/L | India | 2019–2020 | [52] |
| River water | LC-MS/MS | N.D. 1 −135 ng/L Mean 25.4 ng/L |
China | 2018, 2019 and 2021 | [49] |
| River water | Gas chromatography-mass spectrometer (GC-MS) | 0.06–500 ng/L Mean 176.2 ng/L |
Morocco | 2019 | [53] |
| River water | LC-MS/MS | Up to 74.3 µg/L | India | / 2 | [54] |
| River water | 高效液相色谱-串联质谱 (HPLC-MS/MS) | ND 1 -1761 ng/L 季风季节平均 942 ng/L |
印度 | 2018–2019 | [ 55 ] |
| 河水 | 文献资料收集 | ND 1 -293.64 纳克/升 | 中国 | 2010–2019 | [ 56 ] |
| 河水 | 液相色谱-质谱/质谱 | 0.69–17.5 纳克/升 | 中国 | 2019 | [ 48 ] |
| 河水 | 液相色谱-质谱/质谱 | 5.1–874 ng/L 平均 0.06 nM |
加拿大 | 2012–2013 | [ 57 ] |
| 河水 | 高效液相色谱-质谱/质谱 | ND -65.6 ng/L 平均值 0.02 nM |
中国 | 2015 | [ 58 ] |
| 河水 | LC-LC-MS/MS | ND 1 -0.77 nM | 西班牙 | 2012 | [ 59 ] |
| 河水 | 带光电二极管阵列检测的高效液相色谱 | ND 1 -3.87 nM | 南非 | / 2 | [ 60 ] |
| 河水 | 高效液相色谱-质谱/质谱 | 0.349 ± 0.032 纳米 | 英国 | / 2 | [ 61 ] |
| 河水 | 气相色谱-质谱 | 0.01–0.207 海里 | 丹麦 | 2010 | [ 62 ] |
| 海水 | 液相色谱-质谱/质谱 | ND 1 -58.3 ng/L 平均 22.3 ng/L |
中国 | 2018 年、2019 年和 2021 年 | [ 49 ] |
| 海水 | 超高效液相色谱与三重四极杆质谱联用 | ND 1 -8.7 ng/L 平均 4.2 ng/L |
中国 | 2019 | [ 50 ] |
| 地下水 | 液相色谱-质谱/质谱 | 0.5–13.1 ng/L 平均 2.9 µg/L |
波兰 | 2019 | [ 63 ] |
| 饮用水 | 液质联用 | 高达 9.74 纳克/升 | 马来西亚 | 2018 | [ 64 ] |
| 饮用水 | 气相色谱-质谱 | 0.6–9.7 纳克/升 | 中国 | / 2 | [ 65 ] |
1 'ND' 表示未检测到相关数据。 2 '/' 表示未提及相关信息。
目前,还发现 TCS 在藻类、鱼类、鸟类和其他生物体中积累 [ 51 , 66 , 67 ]。帕沙伊等人。[ 28 ] HPLC-MS 发现大西洋鲑、大西洋海狼、虹鳟鱼、大西洋鳕鱼、南美白对虾、印度对虾和小虾等水生生物中 TCS 的浓度为 50-145 μg/kg。在将 WWTP 生物固体应用于农田后,在不同营养水平的农业生态系统中也检测到了 TCS,这意味着 TCS 可以通过陆地食物网转移 [ 31 , 68 , 69]。中国、美国、印度、韩国等国人尿样中均检出TCS,其中中国尿中TCS平均浓度最高(100 ng/mL),越南最低(2.34 ng/mL) ) [ 70 ]。
根据公布的调查数据,TCS是一种在环境中检测频率较高的新兴污染物。低浓度的TCS会影响水生环境中的微生物和鱼类,并可通过食物网传播给人类,对人类生命和健康造成不利影响。因此,有必要研究TCS的降解,特别是其生物降解。
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091713
This entry is offline, you can click here to edit this entry!
