The coronavirus disease 2019 (COVID-19) pandemic has spread worldwide, infecting nearly 500 million people, with more than 6 million deaths recorded globally. Obesity leads people to be more vulnerable, developing worse outcomes that can require hospitalization in intensive care units (ICU). Most studies showed that not only body fat quantity but also its distribution seems to play a crucial role in COVID-19 severity. Compared to the body mass index (BMI), visceral adipose tissue and intrathoracic fat are better predictors of COVID-19 severity and indicate the need for hospitalization in ICU and invasive mechanical ventilation. High volumes of epicardial adipose tissue and its thickness can cause an infection located in the myocardial tissue, thereby enhancing severe COVID-related myocardial damage with impairments in coronary flow reserve and thromboembolism.
- SARS-CoV-2
- COVID-19
- obesity
- body mass index
- intensive care units
1. Introduction
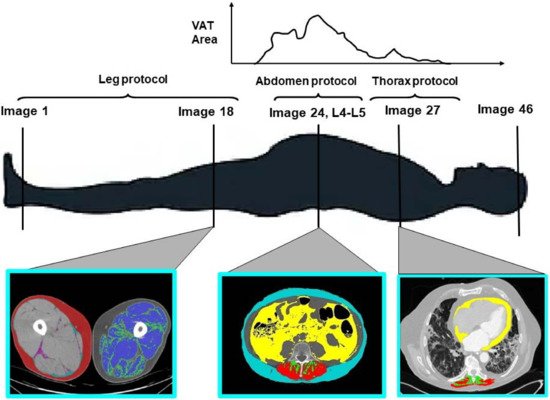
2. The Role of Adipose Tissue Distribution in Patients with Severe COVID-19
2.1. Visceral Adipose Tissue
3. The Role of Skeletal Muscle Mass and Function in SARS-CoV-2 Infection
4. Nutrition in SARS-CoV-2 Prevention and Treatment
4.1. Nutritional Prevention
4.2. Nutritional Risk Assessment
This entry is adapted from the peer-reviewed paper 10.3390/nu14173493
References
- Chen, J. Novel statistics predict the COVID-19 pandemic could terminate in 2022. J. Med. Virol. 2022, 94, 2845–2848.
- El Ghoch, M.; Fakhoury, R. Challenges and New Directions in Obesity Management: Lifestyle Modification Programmes, Pharmacotherapy and Bariatric Surgery. J. Popul. Ther. Clin. Pharmacol. 2019, 26, e1–e4.
- Apovian, C.M. Obesity: Definition, Comorbidities, Causes, and Burden. Am. J. Manag. Care 2016, 22, s176–s185.
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128.
- Ho, J.S.; Fernando, D.I.; Chan, M.Y.; Sia, C.-H. Obesity in COVID-19: A Systematic Review and Meta-analysis. Ann. Acad. Med. Singap. 2020, 49, 996–1008.
- O’Hearn, M.; Liu, J.; Cudhea, F.; Micha, R.; Mozaffarian, D. Coronavirus Disease 2019 Hospitalizations Attributable to Cardiometabolic Conditions in the United States: A Comparative Risk Assessment Analysis. J. Am. Heart Assoc. 2021, 10, e019259.
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Onder, G.; Palmieri, L.; Vanacore, N.; Giuliano, M.; Brusaferro, S.; The Italian National Institute of Health COVID-19 mortality group; Agazio, E.; Andrianou, X.; Barbariol, P.; Bella, A.; et al. Nonrespiratory Complications and Obesity in Patients Dying with COVID-19 in Italy. Obesity 2020, 29, 20–23.
- Rossi, A.P.; Gottin, L.; Donadello, K.; Schweiger, V.; Nocini, R.; Taiana, M.; Zamboni, M.; Polati, E. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by COVID-19. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 762–768.
- Shabanpur, M.; Pourmahmoudi, A.; Nicolau, J.; Veronese, N.; Roustaei, N.; Jahromi, A.J.; Hosseinikia, M. The importance of nutritional status on clinical outcomes among both ICU and Non-ICU patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 225–231.
- Czapla, M.; Juárez-Vela, R.; Gea-Caballero, V.; Zieliński, S.; Zielińska, M. The Association between Nutritional Status and In-Hospital Mortality of COVID-19 in Critically-Ill Patients in the ICU. Nutrients 2021, 13, 3302.
- Khaodhiar, L.; McCowen, K.C.; Blackburn, G.L. Obesity and its comorbid conditions. Clin. Cornerstone 1999, 2, 17–31.
- Cortes-Telles, A.; Ortiz-Farias, D.L.; Pou-Aguilar, Y.; Almeida-De-La-Cruz, L.; Perez-Padilla, J.R. Clinical impact of obesity on respiratory diseases: A real-life study. Lung India 2021, 38, 321–325.
- Colleluori, G.; Graciotti, L.; Pesaresi, M.; Di Vincenzo, A.; Perugini, J.; Di Mercurio, E.; Caucci, S.; Bagnarelli, P.; Zingaretti, C.M.; Nisoli, E.; et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int. J. Obes. 2022, 46, 1009–1017.
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, Fat Mass and Immune System: Role for Leptin. Front. Physiol. 2018, 9, 640.
- Bähr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245.
- Huttunen, R.; Karppelin, M.; Syrjänen, J. Obesity and nosocomial infections. J. Hosp. Infect. 2013, 85, 8–16.
- Falagas, M.E.; Karageorgopoulos, D.E. Adjustment of dosing of antimicrobial agents for bodyweight in adults. Lancet 2009, 375, 248–251.
- Miles, J.; Anderson, D.P.; Engelke, M.; Kirkpatrick, M.K.; Pories, M.L.; Waters, W.G.; Watkins, F.R.; Pokorny, M.E.; Rose, M.A. Barriers to transition of obese patients from hospital to community. Am. J. Manag. Care 2012, 18, e234–e237.
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378.
- Favre, G.; Legueult, K.; Pradier, C.; Raffaelli, C.; Ichai, C.; Iannelli, A.; Redheuil, A.; Lucidarme, O.; Esnault, V. Visceral fat is associated to the severity of COVID-19. Metabolism 2020, 115, 154440.
- Petersen, A.; Bressem, K.; Albrecht, J.; Thieß, H.-M.; Vahldiek, J.; Hamm, B.; Makowski, M.R.; Niehues, A.; Niehues, S.M.; Adams, L.C. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020, 110, 154317.
- Engin, A.B.; Engin, A. Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; Volume 960, ISBN 3-319-48382-X.
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3.
- Gounarides, J.S.; Korach-André, M.; Killary, K.; Argentieri, G.; Turner, O.; Laurent, D. Effect of Dexamethasone on Glucose Tolerance and Fat Metabolism in a Diet-Induced Obesity Mouse Model. Endocrinology 2007, 149, 758–766.
- Rossi, A.P.; Watson, N.L.; Newman, A.B.; Harris, T.B.; Kritchevsky, S.B.; Bauer, D.C.; Satterfield, S.; Goodpaster, B.H.; Zamboni, M. Effects of Body Composition and Adipose Tissue Distribution on Respiratory Function in Elderly Men and Women: The Health, Aging, and Body Composition Study. J. Gerontol. Ser. A 2011, 66, 801–808.
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879.
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234.
- McLeod, M.; Breen, L.; Hamilton, D.; Philp, A. Live strong and prosper: The importance of skeletal muscle strength for healthy ageing. Biogerontology 2016, 17, 497–510.
- Rantanen, T. Muscle strength, disability and mortality: Strengths and disablement. Scand. J. Med. Sci. Sports 2003, 13, 3–8.
- Gariballa, S.; Alessa, A. Impact of poor muscle strength on clinical and service outcomes of older people during both acute illness and after recovery. BMC Geriatr. 2017, 17, 123.
- Guadalupe-Grau, A.; Carnicero, J.A.; Gómez-Cabello, A.; Avila, G.G.; Humanes, S.; Alegre, L.M.; Castro, M.; Rodríguez-Mañas, L.; García-García, F.J. Association of regional muscle strength with mortality and hospitalisation in older people. Age Ageing 2015, 44, 790–795.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Ali, A.M.; Kunugi, H. Skeletal Muscle Damage in COVID-19: A Call for Action. Medicina 2021, 57, 372.
- Ali, A.; Kunugi, H. Physical Frailty/Sarcopenia as a Key Predisposing Factor to Coronavirus Disease 2019 (COVID-19) and Its Complications in Older Adults. BioMed 2021, 1, 11–40.
- Wang, P.-Y.; Li, Y.; Wang, Q. Sarcopenia: An underlying treatment target during the COVID-19 pandemic. Nutrition 2020, 84, 111104.
- Beaudart, C.; Veronese, N.; Sabico, S. Sarcopenia: Research and Clinical Implications; Springer: Berlin/Heidelberg, Germany, 2021.
- Menozzi, R.; Valoriani, F.; Prampolini, F.; Banchelli, F.; Boldrini, E.; Martelli, F.; Galetti, S.; Fari’, R.; Gabriele, S.; Palumbo, P.; et al. Impact of sarcopenia in SARS-CoV-2 patients during two different epidemic waves. Clin. Nutr. ESPEN 2021, 47, 252–259.
- Silva, R.N.; Goulart, C.D.L.; Oliveira, M.R.; Tacao, G.Y.; Back, G.D.; Severin, R.; Faghy, M.A.; Arena, R.; Borghi-Silva, A. Cardiorespiratory and skeletal muscle damage due to COVID-19: Making the urgent case for rehabilitation. Expert Rev. Respir. Med. 2021, 15, 1107–1120.
- Kirwan, R.; McCullough, D.; Butler, T.; de Heredia, F.P.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. GeroScience 2020, 42, 1547–1578.
- Bettis, T.; Kim, B.-J.; Hamrick, M.W. Impact of muscle atrophy on bone metabolism and bone strength: Implications for muscle-bone crosstalk with aging and disuse. Osteoporos. Int. 2018, 29, 1713–1720.
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2021, 21, 614–635.
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181.
- Keusch, G.T. The History of Nutrition: Malnutrition, Infection and Immunity. J. Nutr. 2003, 133, 336S–340S.
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875.
- Li, G.; Zhou, C.-L.; Ba, Y.-M.; Wang, Y.-M.; Song, B.; Cheng, X.-B.; Dong, Q.-F.; Wang, L.-L.; You, S.-S. Nutritional risk and therapy for severe and critical COVID-19 patients: A multicenter retrospective observational study. Clin. Nutr. 2020, 40, 2154–2161.
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 Is Associated with Clinically Significant Weight Loss and Risk of Malnutrition, Independent of Hospitalisation: A Post-Hoc Analysis of a Prospective Cohort Study. Clin. Nutr. 2021, 40, 2420–2426.
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679.
- Yu, Y.; Ye, J.; Chen, M.; Jiang, C.; Lin, W.; Lu, Y.; Ye, H.; Li, Y.; Wang, Y.; Liao, Q.; et al. Malnutrition Prolongs the Hospitalization of Patients with COVID-19 Infection: A Clinical Epidemiological Analysis. J. Nutr. Health Aging 2020, 25, 369–373.
- Földi, M.; Farkas, N.; Kiss, S.; Dembrovszky, F.; Szakács, Z.; Balaskó, M.; Erőss, B.; Hegyi, P.; Szentesi, A. Visceral Adiposity Elevates the Risk of Critical Condition in COVID-19: A Systematic Review and Meta-Analysis. Obesity 2020, 29, 521–528.
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199.
- Mechanick, J.I.; Carbone, S.; Dickerson, R.N.; Hernandez, B.J.; Hurt, R.T.; Irving, S.Y.; Li, D.; McCarthy, M.S.; Mogensen, K.M.; Gautier, J.B.O. Clinical Nutrition Research and the COVID-19 Pandemic: A Scoping Review of the ASPEN COVID-19 Task Force on Nutrition Research. J. Parenter. Enter. Nutr. 2021, 45, 13–31.
- Zampelas, A. Nutritional Habits and Recommendations in the COVID-19 Era. Nutrients 2022, 14, 693.
- James, P.T.; Ali, Z.; Armitage, A.E.; Bonell, A.; Cerami, C.; Drakesmith, H.; Jobe, M.; Jones, K.S.; Liew, Z.; Moore, S.E.; et al. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J. Nutr. 2021, 151, 1854–1878.
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller Vainicher, C.; Rossini, M. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 1968.
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988.
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 58–64.
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638.
- Martindale, R.; Patel, J.J.; Taylor, B.; Arabi, Y.M.; Warren, M.; McClave, S.A. Nutrition Therapy in Critically Ill Patients with Coronavirus Disease 2019. JPEN J. Parenter. Enter. Nutr. 2020, 44, 1174–1184.
- Schueren, M.A.v.B.-d.v.d.; Guaitoli, P.R.; Jansma, E.P.; de Vet, H.C. Nutrition screening tools: Does one size fit all? A systematic review of screening tools for the hospital setting. Clin. Nutr. 2014, 33, 39–58.
- Reignier, J.; Thenoz-Jost, N.; Fiancette, M.; Legendre, E.; Lebert, C.; Bontemps, F.; Clementi, E.; Martin-Lefevre, L. Early enteral nutrition in mechanically ventilated patients in the prone position. Crit. Care Med. 2004, 32, 94–99.
