Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Articular cartilage is a highly organized tissue that provides remarkable load-bearing and low friction properties, allowing for smooth movement of diarthrodial joints; however, due to the avascular, aneural, and non-lymphatic characteristics of cartilage, joint cartilage has self-regeneration and repair limitations. Cartilage tissue engineering is a promising alternative for chondral defect repair.
- fibrin
- articular cartilage
- scaffold
1. Introduction
Articular cartilage is a highly organized tissue that provides remarkable load-bearing and low friction properties, allowing for smooth movement of diarthrodial joints [1]. Joint cartilage contains sparsely distributed chondrocytes embedded within the extracellular matrix (ECM). The ECM is mainly comprised of water, type II collagen, and glycosaminoglycans that provide the tissue with sufficient mechanical properties for several biological functions, such as load-bearing and low friction [2,3]. Due to the avascular, aneural, and non-lymphatic characteristics of cartilage, joint cartilage has self-regeneration and repair limitations [2]. When the cartilage gets damaged, if the diameter of the injury is greater than 4 mm, spontaneous self-repair capacity becomes limited [4]. Moreover, focal cartilage lesions predispose to developing early-onset osteoarthritis, which may lead to long rehabilitation periods and loss of function.
Treatment for cartilage injuries focuses on relieving pain [5]. It may include lifestyle changes, oral anti-inflammatories [6], physical therapy [7], intra-articular injections of hyaluronic acid or steroids [8], bisphosphonates [9], and even surgical interventions such as joint replacement [7]. Cell therapy is currently being proposed as an alternative strategy. In focal cartilage lesions, autologous chondrocyte implantation and mesenchymal stem cells (MSC) seeded onto scaffolds have been used to restore these defects with good results [10]. Other cell lines that have been used to repair focal cartilage lesions by implantation into the lesion site are embryonic stem cells and induced pluripotent stem cells (iPSCs) [11]; however, even though cell therapy has limited therapeutic activity, one of its main advantages is the short-term reduction in clinical symptoms. Thus, medicine has turned to a tissue engineering approach to prolong the therapeutic effect of cell therapy.
The successful use of tissue engineering techniques to form engineered cartilage is based on a combination of three critical elements: a cellular component, a bioactive component (such as growth factors), and a biocompatible and mechanically stable carrier vehicle/matrix scaffold [12]. The cellular component consists of healthy, viable cells that are accessible, manipulable, and nonimmunogenic. The bioactive component should promote the differentiation and maturation of the cellular component. The carrier has a dual function, acting as both a delivery vehicle and a scaffold [13]. In addition, the carrier should provide sufficient mechanical support to withstand in vivo forces [14] and must be degraded by the cells, giving way to its replacement or contributing to the formation of new tissue [15,16,17].
The basic types of biomaterials used In tissue engineering can be classified in relation to their origin as synthetic (usually chemical-nature materials) and natural (derived from biomolecules, tissues, or living organisms) [18] depending on its structural patterns as a polymer (composed of many repeated subunits) and a composite (a combination of two different biomaterials, a polymer, and a filler) [19]. Natural polymers such as collagen, silk fibroin, and fibrin are some of the most common, used as scaffolds for cartilage engineering. Fibrin is one of the most promising natural biomaterials for articular cartilage repair [20]. Fibrin polymers and composites have been used to induce regeneration as a vehicle for bioactive molecules to promote injury healing and delivery carriers for multiple cell lines [21]. Moreover, they are not expensive and are easy to obtain from whole blood.
2. Fibrin: Structure and Molecular Interactions
Fibrin is a native biopolymer derived from fibrinogen [22,23]. Fibrinogen is a blood component that plays an important role in hemostatic function. It is also related to cellular processes such as proliferation, differentiation, adhesion, migration, healing, inflammation, and angiogenesis [24]. Fibrinogen is described as a long glycoprotein (340 Kda) made up of a dimer of three disulfide-linked polypeptide chains called Aα (66,500 Da), Bβ (52,000 Da), and γ (46,500 Da) [22,25]. Fibrinogen consists of two globular D regions and one central globular E region, each with a part of α-helical coiled-coils.
Fibrinogen is transformed into fibrin monomers during blood clotting due to thrombin [23], which cleaves fibrinopeptide A (FpA) and fibrinopeptide B (FpB) from the N-terminal sites of the Aα and Bβ chains of fibrinogen, respectively Figure 1A. At this point the fibrin fibers made of two fibrin nano peptides half-staggered with a crystalline-like structure, can reach a size of 100 nm, After FpAs cleavage each α chains have a new sequence on the N-terminal (Gly-Pro-Arg) called knobs “A” [26], then these fibers can come together and form a crosslinked mesh. This initiates fibrin assembly by exposing a polymerization site called EA. Each EA site combines with a constitutive complementary-binding pocket in the D domain (Da) to form the initial EA:Da association by forming intermolecular ε-((γ-glutamyl) lysine bonds, causing double-stranded twisting fibrils by aligning in a staggered overlapping end-to-middle domain arrangement [27]. The g-g crosslinks form reciprocally between glutamine 398 or 399 and lysine 406, and other flexible bonds form such as a–a crosslinks Gln-221, -237, -328, -366 and Lys-418, -448, -508, -539, -556, -580, and -601, all conferring particular mechanical and elastic properties [28] (Figure 1B). The C-terminal region of each fibrinogen or fibrin c-chain contains one crosslinking site at factor XIII or XIIIa. These give fibrin structural integrity and stabilize the clot against proteolytic and mechanical insults because of isopeptide bond formation, passing from a soluble state to an insoluble one crosslinked by ε-(γ-glutamyl)-lysine stable covalent bonds [25] (Figure 1C).
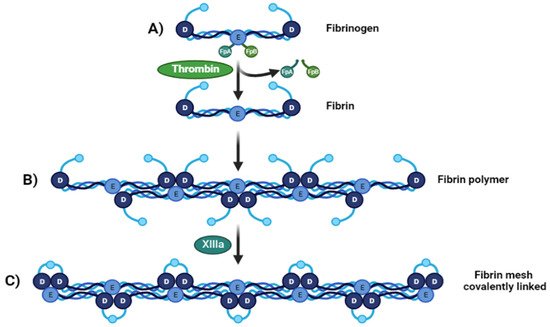
Figure 1. From fibrinogen to fibrin Mesh. (A) Fibrinogen D:E:D regions interact with thrombin-realizing fibrinopeptides (FpA and FpB) (B). Soluble fibrin is then activated by Factor XIIIa, permitting sulfide bonding to crosslink among fibrin, converting it to a (C) crosslinked fibrin polymer.
All the molecular and structural properties mentioned above allow crosslinking of the fibrin with different biomaterials, enhancing fibrin mechanical and elastic properties, and generating new biomaterials and scaffolds that resemble the physical properties of articular cartilage.
3. Mechanical and Physical Properties of the Fibrin Scaffolds
Due to the mechanical resistance, elastic, and mesh-like nature, fibrin has been used as a sealant for surgical procedures and recently as hydrogel scaffold for cartilage engineering. Generally, fibrin scaffolds can be manufactured in three forms: fibrin glues, fibrin hydrogels, and fibrin microbeads [13]. The fibrin glues are obtained from plasma cryoprecipitate (which contains fibrinogen, fibronectin, and XIIIa factor). The cryoprecipitate is mixed with thrombin and calcium to obtain a fibrin polymer, which can be used as a patch (2D) or as a 3D scaffold [29]. Fibrin hydrogels are made from purified fibrinogen, thrombin, and calcium salt. The main difference between fibrin glues and hydrogels is the presence of coagulable proteins in the fibrin glue [30]. On the other hand, fibrin microbeads are obtained from fragmented plasma and thrombin; however, polymerization takes place in an emulsifier at 75 °C where the fibrinogen gets denaturalized and the XVIIIa factor crosslinks the fibrin into a more stable and dense form [13]. In all the fibrin scaffolds, the mechanical strength will depend on the amount of fibrin and thrombin [31]; however, when it comes to fibrin composites, the fibrin becomes a filler and a functional part, and the mechanical strength will depend on the other component or phase of the biomaterial.
To increase their mechanical strength, the fibrin scaffolds have been combined with different biomaterials, such as poly lactic-co-glycolic acid (PLGA) [32,33,34], hyaluronic acid (HA) [35], chitosan–alginate [36], polycaprolactone (PCL) [37], and although the results have been promising, increasing approximately 60 times the fibrin mechanical strength, in some cases, they match articular cartilage (0.24–0.85 Mpa [38]); however, it has not yet been possible to develop a scaffold that can match all the properties of cartilage.
For example, an increase in the amount of fibrin leads to greater mechanical strength, but the pore size decreases (Table 1). Pore size must be appropriate for some cell types; for example, 150–250 µm are desirable for articular chondrocytes [39] and 200–300 µm for MSC [40] to promote cell proliferation and the preservation of chondrogenic differentiation into the scaffold, ensuring the diffusion of oxygen, nutrients, and products of metabolism.
Fibrin combinations with some biomaterials achieve a suitable pore size, but due to the chemical nature of the biomaterial [41], there is a lack of growth factors that can improve or maintain chondrogenesis, so these must be provided to the scaffold formulation [42]. Other combinations have achieved an accurate pore size and mechanical strength, but with a decrease in scaffold elasticity, as shown in Table 1.
Creating a three-dimensional scaffold does not guarantee the creation of high-quality new cartilage tissue on its own. Instead, some help is needed to improve or induce the generation of new tissue. There are also numerous molecular interactions and conditions that contribute to the mechanical resistance of the scaffold. Usually, the scaffold is seeded with a cellular component, and this can improve the mechanical properties through ECM production [43]. Depending on the approach, the cell component in the scaffold should replace part of the scaffold for new tissue. At this point, the ECM can add mechanical support, elasticity, and stiffness [44]. That is why all the properties should be tested with and without cells to know the limitations of scaffolds.
With the creation of different scaffolds for cartilage repair, it is critical to compare their properties to choose a therapeutic approach. However, it has been difficult to compare the properties of the scaffolds due to inconsistency in the number and type of tests performed to prove their functionality. Ideally, a minimum number of tests should be set to evaluate their functionality and compare with others.
Table 1. Physical and mechanical properties of composed scaffold with fibrin.
| Scaffold | Fibrin/Fibrinogen Content (mg/mL) |
Pore Size (µm) |
Mechanical Strength (Mpa) |
Longitudinal Elasticity (Youngs Modulus) |
Reference | ||
|---|---|---|---|---|---|---|---|
| Other Component Content | Elastic Modulus (kPa) |
Elongation at Break (%) |
|||||
| Fibrin glue (Tiseel) | 67–106 | - | - | ≈0.0029 | 15 | - | [45] |
| Fibrin glue (EVICEL) | 55–85 | - | - | 0.0135 | 38 | - | [45] |
| Fibrin hydrogel | 5 | - | 9.7 ± 7.1 | 0.0034 | - | - | [31] |
| Fibrin hydrogel | 12.5 | - | 8.1 ± 5.3 | 0.0054 | - | - | [31] |
| Fibrin hydrogel | 25 | - | 6.4 ± 3.4 | 0.0109 | - | - | [31] |
| Fibrin hydrogel | 50 | - | - | ≈0.01 | 20 | - | [46] |
| Hydrogel: Fibrin-PAAm | 50 | 44.46% PAAm | - | ≈0.052 | 120 | ≈55 | [46] |
| Composite: Fibrin-PAAm-PCL | 44.46% PAAm PCL as a core |
- | ≈0.16 | 150 | ≈22 | [46] | |
| Composite: Fibrin-collagen sponge | 110 | - | ≈110 | ≈12 | - | - | [47] |
| Composite: Fibrin-genipin crosslinked DCM-PVA | - | Genipin = 0.04 g/g DMC-PVA 70:30 |
22–95 | - | 14.7 ± 2.7 | 62.39 ± 6.56 | [16] |
| Htdrogel: Fibrin-PLC-ECM | - | PCL = 28% ECM = 2%, 5%, and 10% |
250–400 | 0.13–0.20 | - | - | [37] |
| Hydrogel: Fibrin-PLC-ECM (salt leached) | - | PCL = 28% ECM = 2%, 5%, and 10% |
<400 | 0.02–0.05 | - | - | [37] |
| Advanced platelet-rich fibrin glue | - | - | - | 0.17 | ≈70 | ≈25 | [48] |
| Platelet.poor plasma-derived fibrin glue | - | - | - | 0.13 | ≈70 | ≈15 | [48] |
poly-ε-caprolactone (PCL); poly-vinyl alcohol (PVA); devitalized cartilage matrix (DCM); extracellular matrix (ECM).
4. Cells on Fibrin Scaffolds
The cell component in the scaffold becomes one of the most important parts because it can improve and accelerate the regeneration process. Additionally, biomaterials cannot only improve the biocompatibility of the scaffold, but also enhance cell culture on the scaffold [78]. Cell–fibrinogen interactions are complex and mediated by the interaction of cell binding receptors (integrin) and surface adhesion sites (ligands) [79]. During wound healing, fibrin clots interact with ECM proteins such as fibronectin and vitronectin [30], creating a network or provisional scaffold binding endothelial cells, leukocytes, platelets and plasma proteins to the clot [80]. Fibrinogen presents sequences recognized by some integrins; for example, RGD (Arg-Gly-Asp). RGD is one of the most extensively studied. It can bind to multiple integrin species; for example, in platelets, RGD binding integrins, including αIIbβ3, αvβ3, and α5β1, by hydrogen bonds. Besides the sterilization process causing ECM protein denaturation, the functionality of the RGD domain is generally preserved, which minimizes the risk of immune reactivity and increases the number of attached cells when the material is used in a scaffold [81].
Fibrin scaffolds have been used for endothelial cells, leukocytes, platelets, and plasma protein binding [80]. In healthy normal adult joint cartilage, chondrocytes are inactive cells that are considered the structural and functional unit. They exhibit low metabolic and very little renewal activity of the matrix components [82]. They are avascular and thus lack nutrients. In tissue engineering, promoting chondrocyte proliferation and maintaining the chondrocyte phenotype are key points, hence several source cells such as differentiated cells (AC; articular chondrocytes, NC; nasal septum chondrocytes), progenitor cells, multipotent cells (MSC derived from bone marrow, adipose tissue, synovial membrane, synovial fluid, umbilical cord blood, and peripheral blood), or pluripotent cells (ESC; embryonic stem cells and induced pluripotent stem cells) are used [83].
Some advantages of stem cells for cartilage lesion repair are infinite proliferation capacity [84] and excreting chondrocyte-promoting growth factors such as fibroblast growth factor-1(FGF-1) and transforming growth factor-β (TGF-β) [85,86]. Different methods, such as physical stimulation, application of growth factors, and peptides have been proposed to favor chondrocyte proliferation [87]. The latter have been categorized as small molecule compounds that can promote the proliferation of chondrocytes and induce stem cell chondrogenesis. Examples of these compounds include kartogenin, melatonin, simvastatin, dexamethasone, prostaglandin E2, and glucosamine. In terms of cell adhesion, it is necessary to consider the hydrophilicity of the materials. Studies have suggested that the encapsulation of chondrocytes in different biomaterials maintains the cartilage phenotype in vitro for long periods of time. The availability and compatibility of donor tissue has been proposed as the main limitation for this type of procedure [88].
For greater biocompatibility, even proliferation of chondrocytes and osteoblasts, modification of the very nature of the biomaterial has been proposed, as is the case of carbon nanofibers that have been proposed as an alternative in the treatment of bone diseases. Such modifications are at the surface level, thanks to sodium hyaluronate, graphene oxide, silica oxycarbide, or by oxidation.
A greater number of osteoblastic cells and chondrocytes are observed after 72 h into modification scaffolds with of sodium hyaluronate because it promotes the migration, proliferation, and even differentiation of bone cells. Sodium hyaluronate together with hyaluronic acid are incorporated into joint cartilage where they have a biological effect on chondrocytes through CD44 receptors. [89]. Adding well-differentiated allogeneic chondrocytes does not exacerbate the mild host tissue reaction caused by scaffolds, especially those made of porous sponge, probably because the newly synthesized pericellular matrix insulates them from the immune reaction [90].
Studies have shown that scaffolds based on native cartilage ECM components and cartilage specific glycosaminoglycans [91] promote the metabolic activity of chondrocytes and the production of ECM. This could enhance the protection of allogeneic cells against an immune system response.
Embryonic stem cells have been shown to differentiate into chondrocytes in a two-step process, where stem cells initially change their phenotype to chondrogenic progenitors, followed by the differentiation of these progenitor cells into chondrocytes. In vitro differentiation of these stem cells is very effective when combined with a three-dimensional microenvironment, with the addition of growth factors to enhance differentiation [92]. Chondrocyte coculture has also been implemented as a method to support chondrogenesis of MSC and maintain their chondrogenicity [93]. iPSCs are an alternative cell source very similar to ESCs. However, there are certain problems with the clinical application of these cells such as inefficient protocols for their differentiation into functional chondrocytes and the risk of residual undifferentiated cells that may have tumorigenic potential.
As discuss, there are advantages and disadvantages according to the different stages of differentiation (for stem cells) or the use of differentiated cells (such as chondrocytes) in the scaffold in terms of its clinical use. Collection, availability, proliferation, chondrogenic potential, ethical and safety considerations, and the potential for allogeneic or autologous uses are the most important aspects [83]. However, it is important to define the number of cells implanted in the fibrin scaffold, because unlike stem cells, chondrocytes have an accelerated rate of degradation due to their capacity to generate ECM.
Other promising sources of adult stem cells to achieve cartilage regeneration are MSCs; which are not as homogeneous as differentiated cells but can be collected from many tissues (with variations in abundance and chondrogenic potential), thus requiring optimization and adaptation of protocols, as well as a complete characterization. To date, the most favorable results are obtained with bone marrow mesenchymal stem cells (BM-MSC); nevertheless, to enhance the viability, function, and in some cases, achieve the differentiation of the cell seeded into some scaffolds, some growth factors are required.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23179879
This entry is offline, you can click here to edit this entry!
