Calcium phosphate nanocluster complexes comprise a core of amorphous calcium phosphate and a sequestering shell of intrinsically disordered phosphopeptides or phosphoproteins. Solutions containing the nanocluster complexes can be thermodynamically stable or metastable due to a tendency to form a precipitate enriched in calcium phosphate. Theoretical and biophysical studies with native and recombinant phosphopeptides have shown how the radius of the core and the stability of the solution depend on the concentration of the sequestering peptide, its affinity for the calcium phosphate and its concentration in relation to the concentration of the calcium phosphate. The thickness of the sequestering shell depends on the conformation of the peptide on the core surface. A sequestering peptide is a flexible sequence including one or more short linear motifs, each of which usually contains several phosphorylated and other acidic residues. These are the main binding sites to the core so that a peptide with several binding motifs can forms loops and trains on the core surface. Calcium phosphate nanocluster complexes were first identified as substructures of casein micelles in milk and have been prepared as individual particles from peptides derived from caseins and osteopontin. Stable biofluids containing nanocluster complexes cannot cause soft tissues to become mineralized whereas stable or metastable biofluids containing nanocluster complexes can help to mineralize hard tissues.
- Calcium phosphate
- biocalcification
- phosphoprotein
- serum
- milk
- saliva
- urine
- small angle neutron scattering
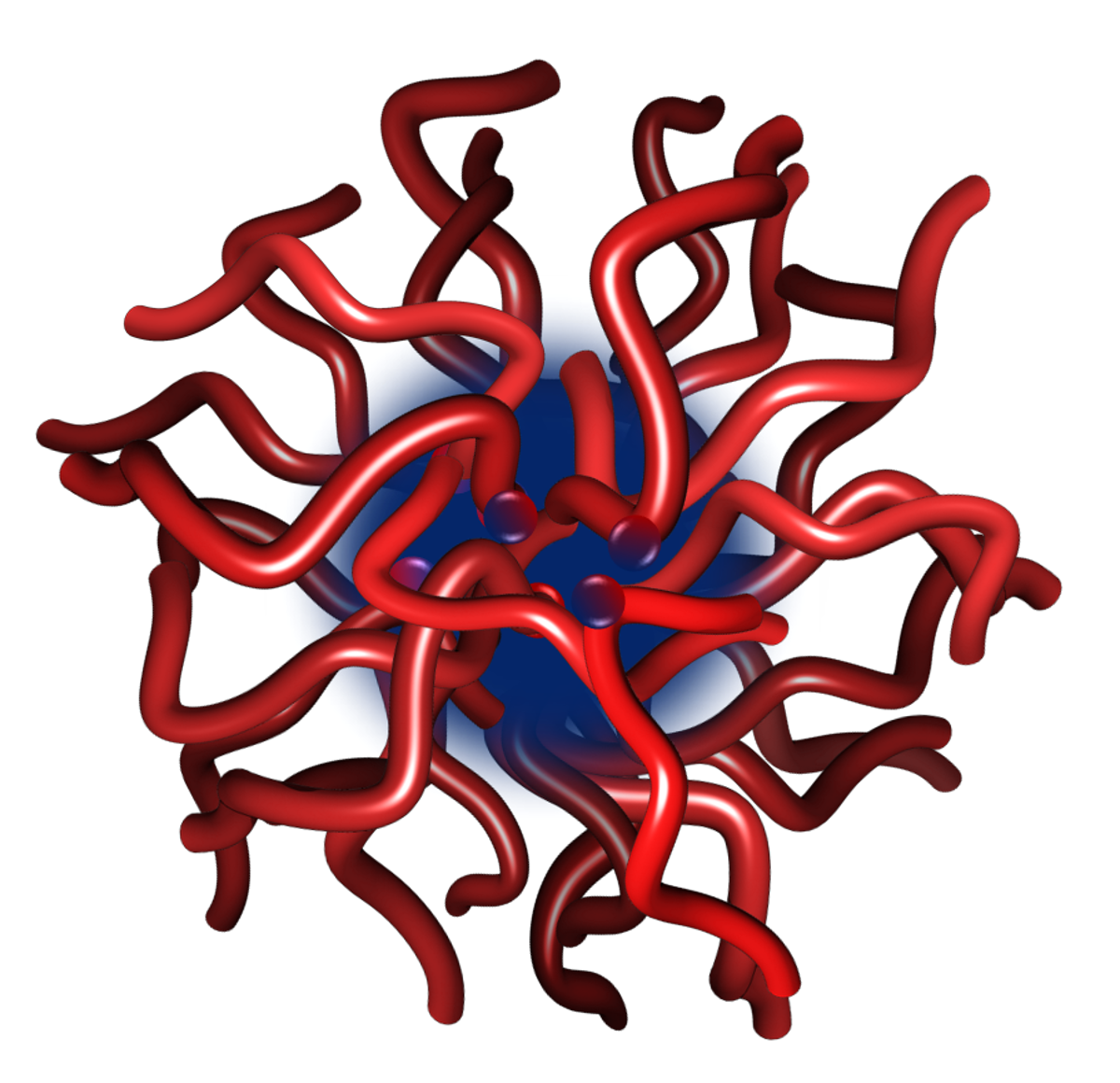
Figure 1. Representation of a calcium phosphate nanocluster complex as a core-shell structure comprising a core of several hundred molecules of amorphous calcium phosphate surrounded by a sequestering shell of several tens of phosphopeptides or phosphoproteins.
A typical calcium phosphate nanocluster complex (Figure 1) comprises a core of several hundred molecules of amorphous calcium phosphate and a few tens of sequestering phosphoproteins or phosphopeptides. Solutions containing the nanocluster complexes can be either thermodynamically stable or may have a tendency to form a calcium phosphate-rich precipitate over time so they can be described as metastable or unstable, depending on the degree of instability. The core radius, chemical composition and shell thickness depend on the solution conditions and nature of the phosphopeptide. Sequestering phosphopeptides are intrinsically disordered and contain one or more short linear motifs, called CaP-SLiMs, which are the primary sites of interaction between the peptide and the core.
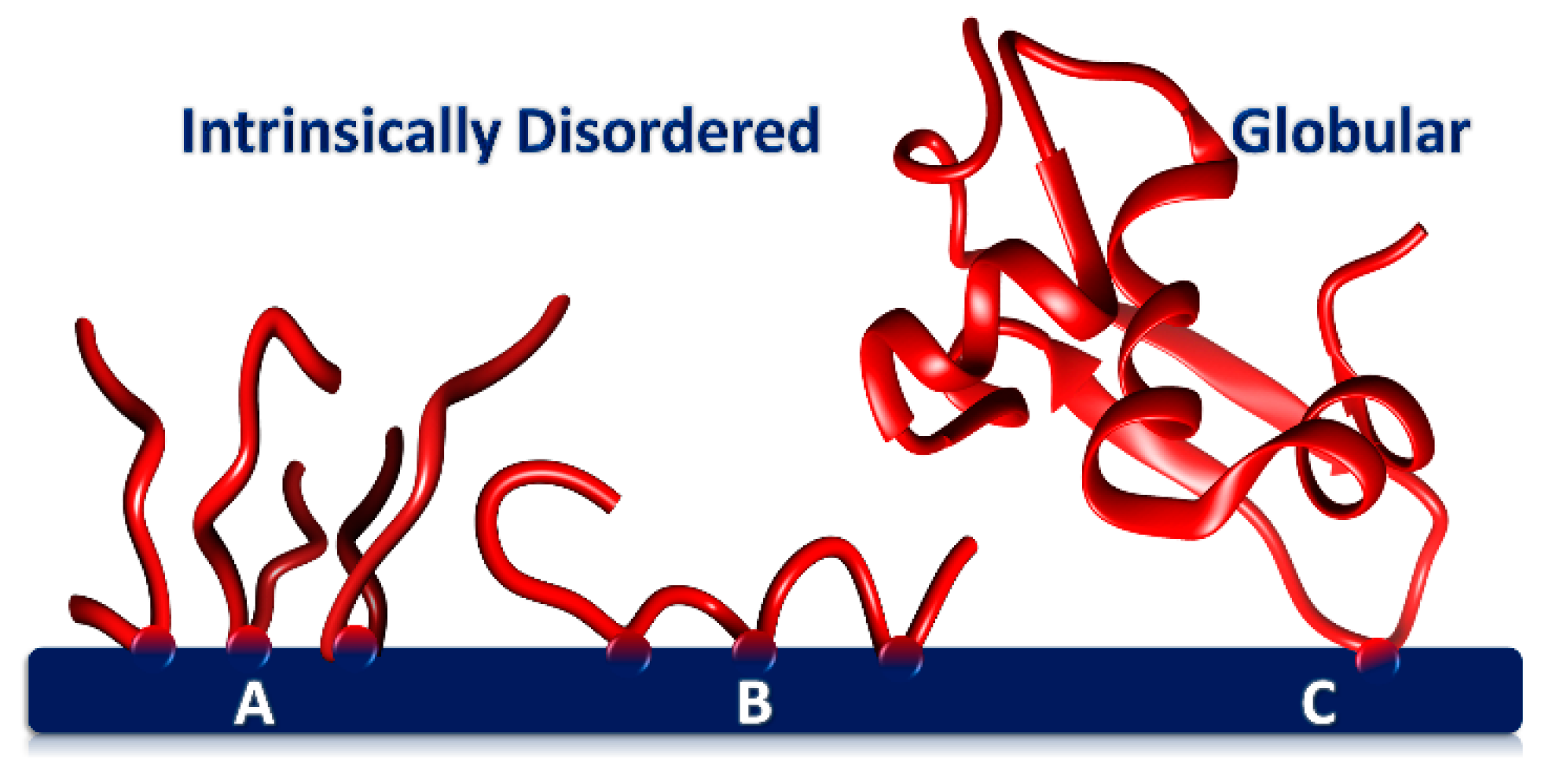
Figure 2. Phosphoproteins adsorbed onto a CaP surface through one or more CaP-SLiMs. (A) Tightly packed layer of flexible, short acidic peptides with a single CaP-SLiM (B) IDP with three CaP-SLiMs (e.g., osteopontin1–149) forming two loops and two trains. A protein such as phosphophoryn has many CaP-SLiMs and as a result adopts a more sessile conformation on the surface. (C) Globular protein adsorbed through a single CaP-SLiM but with a large footprint.
The conformation of the phosphopeptide on the core surface therefore depends on the number of CaP-SLiMs per peptide. Figure 2 shows how peptides with a single CaP-SLiM can pack closely together on the core surface (A) or, if there is more than one CaP-SLiM, form loops and trains on the core surface (B). Peptide flexibility appears to be important in allowing a higher density of packing on the core surface than can be achieved with a globular protein (C).
Calcium phosphate nanocluster complexes were first identified as substructures of casein micelles in milk. It should be stressed that casein micelles have no similarity to soap micelles. They comprise a polydisperse distribution of casein proteins in which about 7% of the dry weight is calcium phosphate, originally called colloidal calcium phosphate. A typical casein micelle has a radius of 70 to 80 nm and contains about 400 of the nanocluster complexes [1]. Further understanding of their composition, structure, solubility and other physico-chemical properties resulted from the preparation of artificial nanocluster complexes in the laboratory from casein[2][3][4][5][6], osteopontin[7][8][9][10] or recombinant phosphopeptides[11].
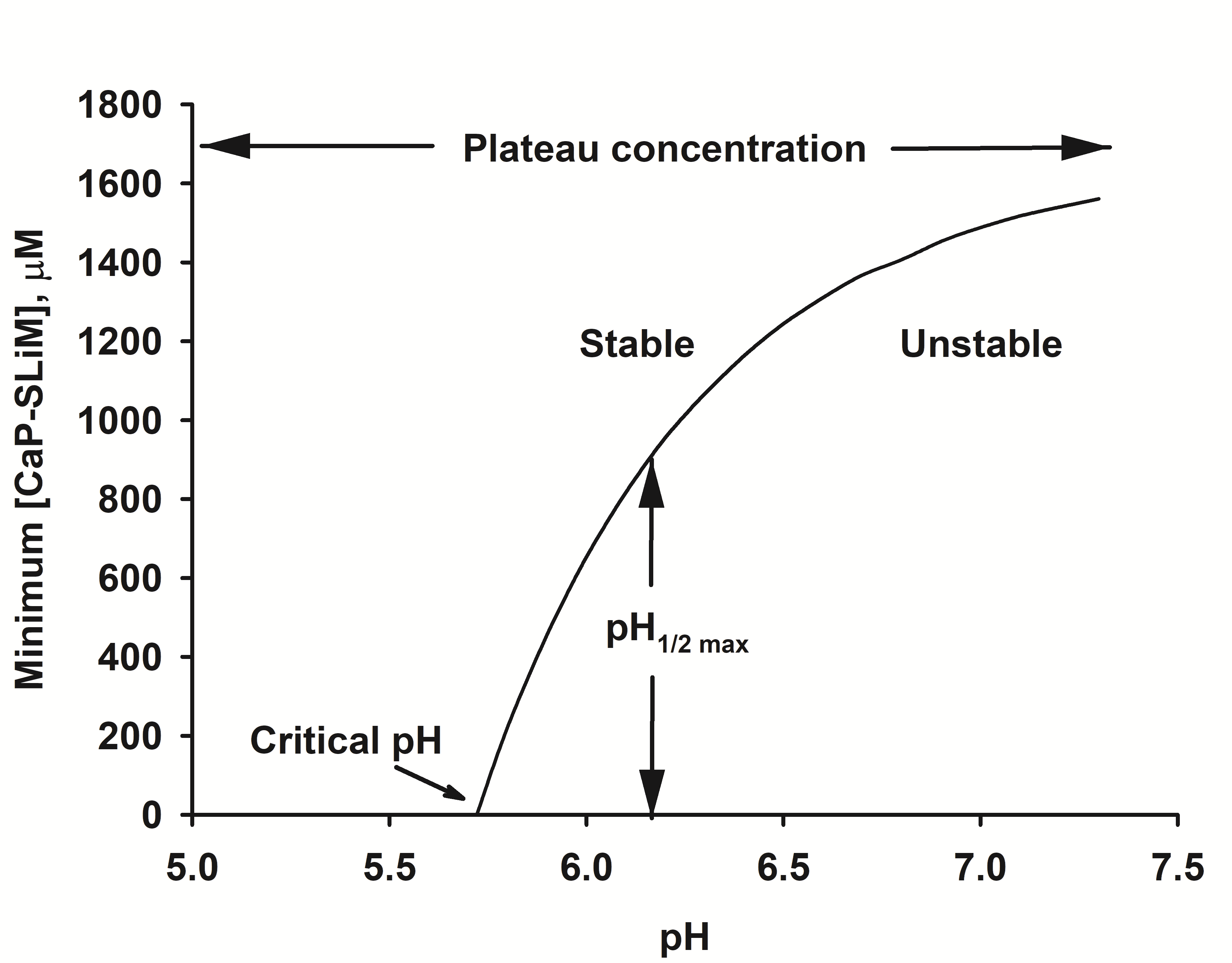
Figure 3. Stability diagram of a solution of calcium phosphate nanocluster complexes. The solution used for the diagram has the salt composition of a typical bovine milk. Below the critical pH no phosphopeptide is needed for stability. Above the critical pH the solution is either stable or metastable, depending on the concentration of calcium phosphate-binding motifs, [CaP-SLiMs]. If the concentration of calcium phosphate binding motifs is above a plateau level, the solution is stable at any pH.
Detailed physico-chemical measurements of the composition and solubility of the calcium phosphate in solutions containing the artificial nanocluster complexes have been made to allow a fairly complete and quantitatively precise model of the equilibria among the ions and proteins to be constructed. Among the findings is a relationship between the activities of the ions in the continuous phase which gives rise to an invariant ion activity product. The invariant ion activity product is analogous to the solubility constant that is present at equilibrium between an ionic macroscopic phase and a saturated solution of the constituent ions. Application of the model to milk[12] gives accurate predictions of the partition of salts between the casein micelles and the continuous phase. The same type of calculations have been made for some other biofluids[13]. Some biofluids show an invariant ion activity product closely similar to that of a solution of nanocluster complexes formed with osteopontin phosphopeptides.
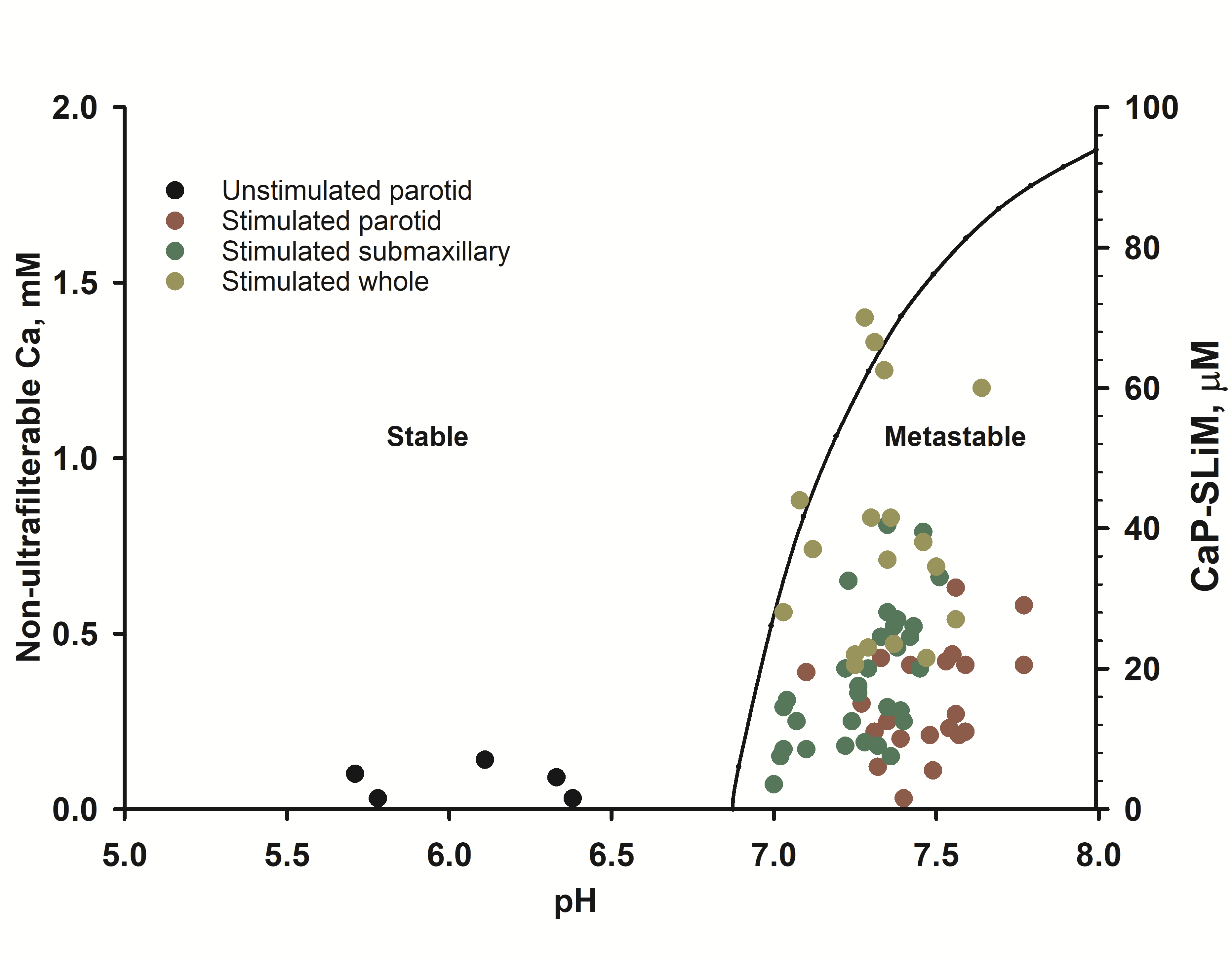
Figure 4. Stability diagram of stimulated human saliva (right-hand axis) superimposed on experimental compositional data (left hand axis) on stimulated and unstimulated saliva showing that the latter is metastable. Taken from Figure 12 of Lenton et al.[14].
In human saliva, most of the calcium phosphate binding motifs are on statherin. Figure 4 shows the stability diagram for stimulated saliva showing that the critical pH is below the physiological pH of stimulated saliva but below that of resting saliva. The concentration of statherin in the samples shown in Figure 4[15] was not measured but if they are typical of other reported concentrations[16] then most stimulated saliva samples fall in the metastable part of the stability diagram.
In the review of Lenton et al.[14] physico-chemical principles governing the formation, composition, size, structure, and stability of the nanocluster complexes are described. Examples are given of complexes formed by casein, osteopontin, and recombinant phosphopeptides. Application of these principles and properties to blood serum, milk, urine, and resting saliva is described to show that under physiological conditions they are in the stable region of their stability diagram and so cannot cause soft tissue calcification. Stimulated saliva, however, is in the metastable region, consistent with its role in tooth remineralization. Destabilization of biofluids, with consequential ill-effects, can occur when there is a failure of homeostasis, such as an increase in pH without a balancing increase in the concentration of sequestering phosphopeptides.
Many organisms contain soft and mineralised tissues in intimate contact with each other, some, apparently permeated by the same biofluid. The periodontal ligament is a perfect example. It is a soft tissue lying between two highly mineralized tissues, bone and cementum, but is resistant to mineralization[19]. The importance of phosphoproteins in maintaining a balance between the tissue types (Figure 5) has long been realized[17][18]. Biofluids that contain stable calcium phosphate nanoclusters sequestered by phosphopeptides make it possible for soft and hard tissues to co-exist in the same organism with relative ease. In the stable region of the stability diagram of a biofluid, amorphous calcium phosphate cannot precipitate. because the solution lies in a local free energy minimum. Nevertheless, if the solution is brought into contact with hydroxyapatite, the crystalline phase will grow at the expense of the nanocluster complexes because it is thermodynamically more stable. High concentrations of calcium and phosphate can be brought to the mineralization front of a hard tissue in the form of stable calcium phosphate nanocluster complexes.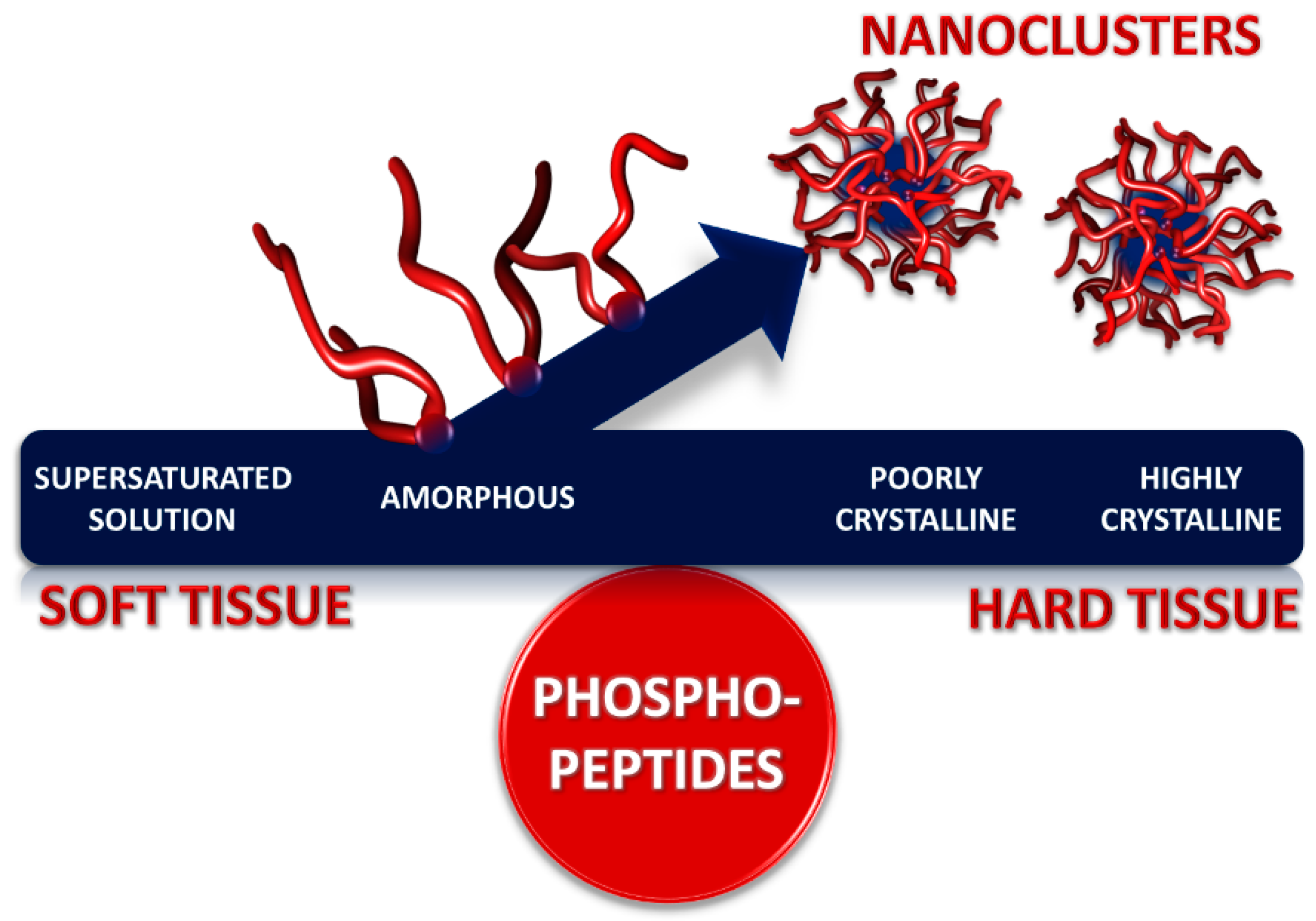
This entry is adapted from the peer-reviewed paper 10.3390/cryst10090755
References
- John A. Carver; Carl Holt; Functional and dysfunctional folding, association and aggregation of caseins. Advances in Protein Chemistry and Structural Biology 2019, 118, 163-216, 10.1016/bs.apcsb.2019.09.002.
- Carl Holt; Magnus N. Wahlgren; Torbjörn Drakenberg; Ability of a β-casein phosphopeptide to modulate the precipitation of calcium phosphate by forming amorphous dicalcium phosphate nanoclusters. Biochemical Journal 1996, 314, 1035-1039, 10.1042/bj3141035.
- Carl Holt; Peter A. Timmins; Neil Errington; Jeffrey Leaver; A core-shell model of calcium phosphate nanoclusters stabilized by beta-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements.. European Journal of Biochemistry 1998, 252, 73-78, 10.1046/j.1432-1327.1998.2520073.x.
- Elaine M. Little; Carl Holt; An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein phosphopeptides. European Biophysics Journal 2004, 33, 435-447, 10.1007/s00249-003-0376-x.
- Keith J. Cross; N Laila Huq; Eric C. Reynolds; Casein Phosphopeptide–Amorphous Calcium Phosphate Nanocomplexes: A Structural Model. Biochemistry 2016, 55, 4316-4325, 10.1021/acs.biochem.6b00522.
- Keith J Cross; N Laila Huq; David Stanton; M. Sum; Eric C. Reynolds; NMR studies of a novel calcium, phosphate and fluoride delivery vehicle-αS1-casein(59–79) by stabilized amorphous calcium fluoride phosphate nanocomplexes. Biomaterials 2004, 25, 5061-5069, 10.1016/j.biomaterials.2004.01.045.
- Carl Holt; Esben S. Sørensen; Roger A. Clegg; Role of calcium phosphate nanoclusters in the control of calcification. The FEBS Journal 2009, 276, 2308-2323, 10.1111/j.1742-4658.2009.06958.x.
- Samuel Lenton; Tilo Seydel; T. Nylander; C. Holt; M. Härtlein; Susana Teixeira; G. Zaccai; Dynamic footprint of sequestration in the molecular fluctuations of osteopontin. Journal of The Royal Society Interface 2015, 12, 20150506, 10.1098/rsif.2015.0506.
- Samuel Lenton; Tommy Nylander; Carl Holt; Lindsay Sawyer; Michael Härtlein; Harrald Müller; Susana Teixeira; Structural studies of hydrated samples of amorphous calcium phosphate and phosphoprotein nanoclusters. European Biophysics Journal 2016, 45, 405-412, 10.1007/s00249-015-1109-7.
- Samuel Lenton; Marco Grimaldo; Felix R.-Runge; Frank Schreiber; Tommy Nylander; Roger Clegg; Carl Holt; Michael Härtlein; Victoria Sakai; Tilo Seydel; et al. Effect of Phosphorylation on a Human-like Osteopontin Peptide. Biophysical Journal 2017, 112, 1586-1596, 10.1016/j.bpj.2017.03.005.
- Roger A. Clegg; Carl Holt; An E. coli over-expression system for multiply-phosphorylated proteins and its use in a study of calcium phosphate sequestration by novel recombinant phosphopeptides. Protein Expression and Purification 2009, 67, 23-34, 10.1016/j.pep.2009.04.004.
- Etske Bijl; Thom Huppertz; Hein Van Valenberg; Carl Holt; A quantitative model of the bovine casein micelle: ion equilibria and calcium phosphate sequestration by individual caseins in bovine milk. European Biophysics Journal 2018, 48, 45-59, 10.1007/s00249-018-1330-2.
- Carl Holt; Samuel Lenton; Tommy Nylander; Esben S. Sørensen; Susana Teixeira; Mineralisation of soft and hard tissues and the stability of biofluids. Journal of Structural Biology 2014, 185, 383-396, 10.1016/j.jsb.2013.11.009.
- Samuel Lenton; Qian Wang; Tommy Nylander; Susana Teixeira; Carl Holt; Structural Biology of Calcium Phosphate Nanoclusters Sequestered by Phosphoproteins. Crystals 2020, 10, 755, 10.3390/cryst10090755.
- P. Grøn; Saturation of human saliva with calcium phosphates. Archives of Oral Biology 1973, 18, 1385-1392, 10.1016/0003-9969(73)90112-x.
- D.I. Hay; S.K. Schluckebier; E.C. Moreno; D.J. Smith; Basic Biological Sciences Relationship between Concentration of Human Salivary Statherin and Inhibition of Calcium Phosphate Precipitation in Stimulated Human Parotid Saliva. Journal of Dental Research 1984, 63, 857-863, 10.1177/00220345840630060901.
- Anne George; Arthur Veis; Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chemical Reviews 2008, 108, 4670-4693, 10.1021/cr0782729.
- Willi Jahnen-Dechent; Andrea Büscher; Sina Köppert; Alexander Heiss; Makoto Kuro-O; Edward R. Smith; Mud in the blood the role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. Journal of Structural Biology 2020, 212, 107577, 10.1016/j.jsb.2020.107577.
- Qiliang Zuo; Jiangwu Yao; Shifeier Lu; Zhibin Du; Shuigen Li; Feng Lin; Wei Shi; Yufeng Zhang; Yin Xiao; Shuiigen Li; et al. The role of organic phosphate in the spatial control of periodontium complex bio-mineralization: an in vitro study. Journal of Materials Chemistry B 2019, 7, 5956-5965, 10.1039/c9tb01261c.