Thermo-electrochemical cells (also known as thermocells; TECs) represent a promising technology for harvesting and exploiting low grade waste heat (< 100-150ºC) ubiquitous in the modern environment. Based on temperature dependent redox reactions and ion diffusion, emerging liquid-state thermocells convert waste heat energy into electrical energy generating power at low costs, minimal material consumption and negligible carbon foot-print. Highest values of output power and cell potentials have been achieved for the redox ferri/ferrocyanide system and Co2+/3+, with great opportunities for further development in both aqueous and non-aqueous solvents. New thermoelectric applications in the field include wearable and portable electronic devices in the health and performance monitoring sectors; using body heat as a continuous energy source, thermoelectrics are being employed for long-term, continuous powering of these devices. Energy storage in the form of micro supercapacitors and in lithium ion batteries is another emerging application. For waste heat conversion (WHC) to partially replace fossil fuels as an alternative energy source, power generation needs to be commercially viable and cost-effective. Achieving greater power density and operations at higher temperatures will require extensive research and significant developments in the field.
- Thermo-electrochemical cells
- energy harvesters
- low grade waste heat
- wearable electronics
- micro supercapacitors
- thermoelectrics
1. Introduction
The conversion of energy from primary energy sources to their final use is accompanied by several losses in the form of waste heat. Forman et al. [1] have estimated the waste heat potential in transport, commercial, industrial and residential sectors. Nearly 72% of the primary energy consumed was found to be lost as waste heat, of which ~63% of waste heat streams occurred at temperatures below 100 °C. In the USA, conventional industries and power plants are known to annually waste over 8000 TWh as low-grade heat [2]. This ubiquitous waste heat energy in the form of vehicle exhaust, industrial waste heat, geothermal heat, body heat, etc., is found distributed almost everywhere. However, vast amounts of low-grade heat is mostly discarded and rarely exploited commercially due to its intrinsic low temperatures, space-time variations and the lack of cost-effective and efficient energy-recovery technologies [3]. Renewable energies such as solar, wind or nuclear, which have a negligible carbon footprint compared to non-renewable fossil fuels, [4] are seeing a great resurgence.
The transformation of thermal gradients into electrode potentials for generating electricity has been investigated for a long time [5]. The generation of electric potential in the presence of a temperature gradient between different electrical conductors/or semiconductors was discovered by Thomas Johann Seebeck in 1821. Seebeck coefficients, representing the potential difference generated per unit temperature difference, are typically in the order of few µV/K for devices based on semiconductor materials [6]. Early research was mainly in the field of solid-state thermoelectric generators (TEGs) consisting of n- and p- type semiconductors connected in series as modules and then connecting a number of modules in parallel between the heat source and a cold sink. Under the influence of thermal gradients, mobile charge carriers ‘electrons/holes’ diffused from the hot electrode to the cold, building up charges and a small potential difference [7].
Early studies, which later led to thermoelectrochemical converters, were used for applying thermal corrections to electrochemical processes in the field of current sources and in the production of galvanic coatings. However, the gradual dissolution of anodes was found to limit their commercial application [8]. Landry [9] suggested the integration of low- and high-temperature processes to minimize heat losses. Wakao and Nozo [10] developed a process for recovering thermal energy from low-level heat generated during the mixing of nitric acid with water. The advent of nano-structured thermoelectrics has led to a great deal of interest in waste-heat conversion (WHC) to electricity [11]. Although early research was dominated by thermoelectrics, a number of articles have been published in leading journals on new types of WHC devices using a wide range of phenomena. These include thermomagnetic generators [12], ionic heat-to-electricity conversion [13], thermo-osmotic systems [2], liquid-state thermocells [14], high temperature pyroelectric systems [15][16] and organic Rankin cycles [17] for applications in industries, construction, transportation and energy sectors. These devices are used for harvesting energy from a heat source such as automotive exhaust systems [18], fuel cells [19] or hypersonic engines [20]. Such energy harvesting can reduce the charging requirements on batteries while eliminating wired power connections. Most R&D in this field is focussed on developing better n-type and p-type thermoelectric materials.
Thermoelectrochemical (TEC) cells or ‘thermocells’ are an alternative device design based on redox-active electrolytes which can produce much higher potential differences (mV/K vs. µV/K for solid-state semiconductor devices). A thermocell has two identical electrodes in contact with a redox couple electrolyte in the cell and an external connection [21]. Under a thermal gradient, the redox reaction causes the oxidation of the redox couple at the anode and reduction at the cathode. The reduced species are transported back to the anode through diffusion, convection and migration in the electrolyte, creating a continuous reaction and current flow. Within the degradation constraints of cell materials, thermocells are capable of continuously generating electricity without consuming materials or producing emissions.
The following sections present an in-depth overview on the advances made in TEC design and performances focusing of different aspects, new growth areas and emerging concepts in the choice of redox couples, electrolytes, electrode design and configurations. The article is organized as follows. A brief summary of historical developments in the field is provided in Section 2. Sections 3 and 4 highlight developments in redox couples, electrolytes and electrodes spanning several research directions. Emerging applications of thermocells in the fields of wearable and portable devices, energy storage and associated applications are presented in Section 5. The article concludes with highlighting economic concerns and future perspectives.
2. Historical Background
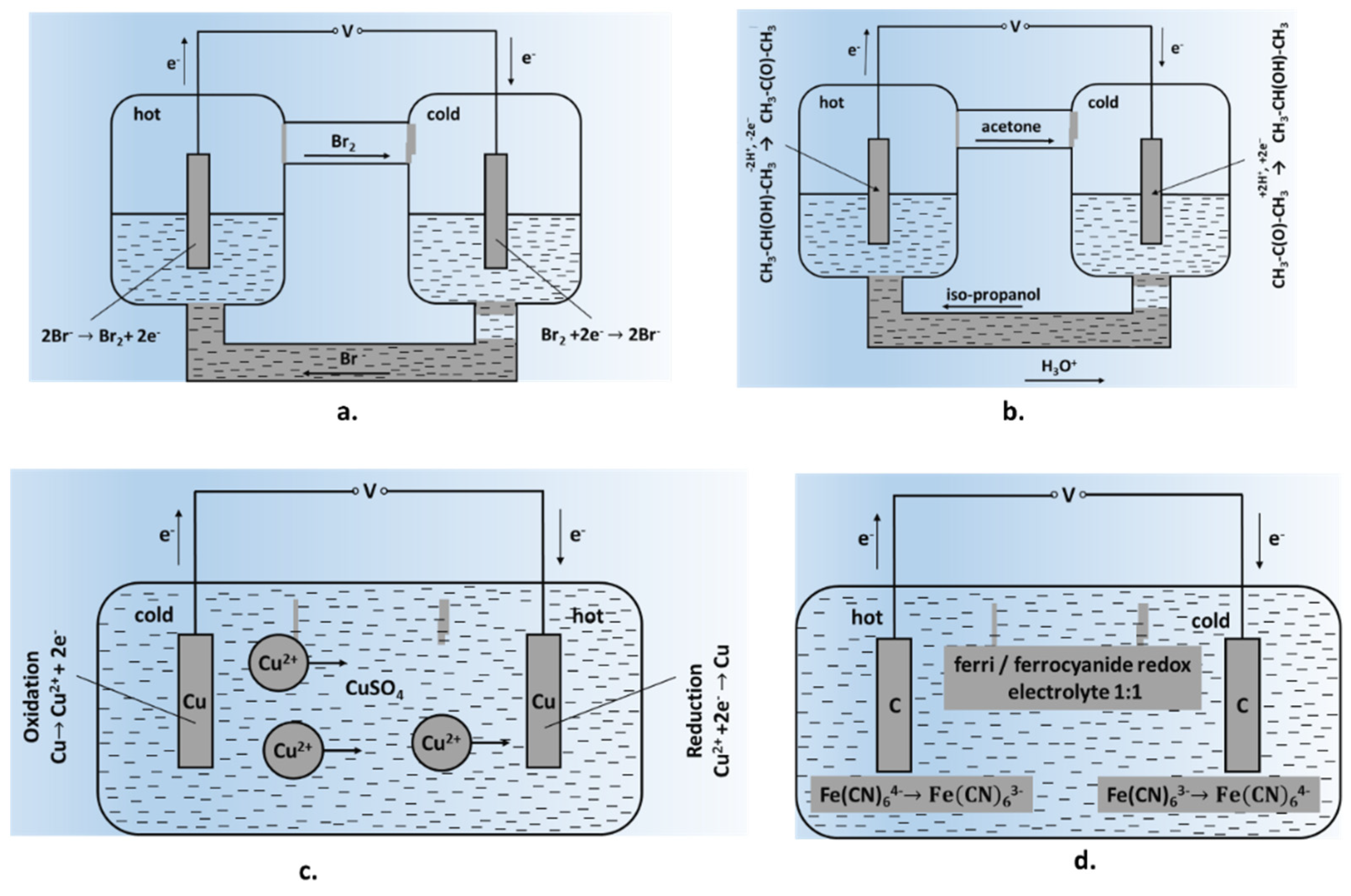
Some of the early-generation TEC cells involved the gradual dissolution of electrodes, limiting their long-term prospects. One such example is shown in Figure 1c, consisting of soluble metal (Cu) electrodes placed in CuSO4 solution at two different temperatures. Such a cell will work until there is a certain degree of electrode consumption, then require a reversal of the temperature gradient for operating in the opposite direction. A significant development was the use of inert electrodes, providing surfaces for released gaseous products such as NO/N2O4/HNO2 or Br−; the released volatile products were condensed at the counter electrode, where the reverse reaction occurred [23][25]. While such processes permitted relatively long and continuous generation of the potential difference as well as higher output power values, these systems had limited stability along with the continuous release of noxious gases. The next stage involved the utilization of redox electrolytes, providing relatively fast kinetics of the electrode process and high values of the hypothetical Seebeck coefficient. Systems based on potassium hexacyanoferrates/ferrites were the most widespread [26]; a schematic representation of this cell is given in Figure 1d. As this system has a negative Seebeck coefficient (−1.4 mV/K), oxidation takes place at the hot electrode and reduction at the cold electrode. At the hot electrode, Fe (CN)64− loses an electron and transforms into Fe (CN)63− which diffuses back towards the cold electrode. At the cold electrode, Fe (CN)63− picks up an electron to transform into Fe(CN)64−, which then diffuses towards the hot electrode thereby setting up a perpetual motion of ions and the generation of electric current. It can be seen that the electrodes remained inert during the process and were not consumed. There has been a significant progress in the cell design and yields since early studies on this system.
Several other types of thermoelectric cells have been developed. These are briefly listed below:
-
TEC cells with phase transitions during mass transfer have been studied for a relatively long time (the first publications date back to the 1970s), but these have not found practical application due to their complexity of design and rapid degradation of components. Wang et al. [27] have recently developed electrochemical sodium heat engines based on phase-change reactions. Nevertheless, their important advantage is their continuous operation, which does not require changing hot and cold sources.
-
Typical TECs are non-isothermal electrochemical cell systems consisting of two electrodes, an electrolyte and a separator. The conversion of thermal energy into electrical energy involves electrode kinetics, thermodynamics, and heat and mass transfer. TEC cells involve changes in the aggregate state of oxidized and reduced forms (nitric acid and nitrogen oxides or KBr and Br2) during continuous operation [8][28].
-
Thermally regenerative electrochemical cycles (TREC) consist of two electrodes with opposite thermopowers; anodes and cathodes generally have positive and negative thermopowers, respectively. For negative thermopower, the cycle involves cooling, discharging, heating and charging; for positive thermopower, the cycle involves heating, charging, cooling and discharging [29].
-
Other cell configurations include thermogalvanic cells (TGC) based on soluble, reversible metal electrodes in solutions of their own salts at different temperatures—TECC cells, using inert electrodes placed in a redox electrolyte or ionic liquid; TGC-Li cells, based on a Li+/Li redox system; TRABs (thermally regenerative ammonia-based batteries); DTCCs (direct thermal charging cells); etc. [30][31][32][33].
3. Electrode Materials and Design
Due to their non-reactive and thermally stable nature, platinum electrodes were used during early studies on thermocells. The electrolyte had to be stirred continuously to achieve Carnot efficiencies of 1.2% [34]. High power generation in thermocells requires large current densities, which may be achieved through high concentrations of redox mediators, increasing the number of sites for redox reactions and exposing electrodes to large thermal gradients [35]. Different types of thermocell electrodes and materials are detailed next.
Reversible metal electrodes: Thermocells using ‘reversible electrodes’ consist of soluble metal electrodes placed in solutions of corresponding salts at two different temperatures (Figure 1c). This cell can only work until a certain level of electrode consumption is reached; the direction of the temperature gradient is then reversed for the cell to work in the opposite direction. Most of the studies have reported on the Cu2+/Cu system due to its simple design and good reproducibility. Seebeck coefficients for this system could be modified from hypothetical values of 0.879 mV/K to 0.75–1.32 mV/K by changing experimental conditions and electrolyte additives [36]. Natural convection was found to have a significant influence on the efficiency of Cu/Cu2+-based thermogalvanic cells. Burmistrov et al. [37] have reported on the efficiency of thermocells based on copper, zinc and nickel metallic electrodes. While the efficiency parameters for Cu electrodes agreed well with the theoretical data and published results, the efficiency of zinc-based TEC cells was somewhat lower than theoretical expectations. The thermogalvanic system with nickel electrodes in an aqueous solution of nickel sulphate exhibited an unusual behaviour.
Inert carbon electrodes: Carbon-based electrodes are becoming increasingly important as a promising and affordable alternative to platinum electrodes. Nanostructured carbon materials, such as single (SWNT) and multiwalled carbon nanotubes (MWNT) and graphene, have a large surface area, which helps in increasing the number of reaction sites. Depending on the number of nanotube walls, the specific surface area of CNTs lies in the range 50–1315 m2/g [38]. These materials also show fast electron-transfer kinetics for the ferrite/ferrocyanide redox couple. Both of these properties can increase the current density achieved with the thermocouple [39].
Hollow nickel microsphere electrodes: Burmistrov et al. [40] have shown that hollow Ni microspheres can be an effective electrode material for thermoelectrochemical cells and provide extremely high values of the hypothetical Seebeck coefficient and open circuit voltage. Electrodes were prepared by pressing tablets of nickel microspheres and reduced at 250 °C. The most effective composition, a KOH-based alkaline electrolyte, was chosen based on the influence of the alkali content on the hypothetical Seebeck coefficient value and specific power as determined by the number of charge carriers in the electrolyte and the intensity of reactions at the electrodes [41][42].
4. Emerging Applications
The conversion of waste heat to electrical energy is finding application in several fields. Some of the emerging applications are presented in this section. While some of these are stand alone on TE effect, others are in conjunction with other technologies.
Wearable and portable devices: Wearable electronic devices are gaining attention in the health and performance-monitoring sectors towards long-term, continuous, self-powered operations using the human body as a continuous supply of energy. The harvesting of body heat in wearable devices has been investigated extensively as an alternative to bulky batteries that require frequent charging or replacement [43][44]. As the core body temperature is regulated at 37 °C, body heat can be a continuous source of energy; the total heat dissipated from the human body can range between 60 to 180 W depending on the activity level [45]. As only a small fraction of the body’s surface can be covered by TEG devices, overall efficiencies are likely to be quite small. Of the several approaches used for harvesting body heat such as electrostatic, piezoelectric, electromagnetic, pyroelectric and thermoelectric effects, the focus will be on thermoelectric mechanisms [46]. Wearable electronic devices find application in medical devices (real-time monitoring, blood pressure sensors, ear-ware), smart watches, sportswear, wristbands and flexible devices for monitoring non-flat surfaces in industrial applications [47].
In rigid TEGs, n- and p-legs and interconnects are typically affixed to a thermally conductive ceramic substrate (alumina or aluminium nitride). In flexible TEGs, gallium-indium eutectic alloys have been used as flexible liquid–metal interconnects to ascertain integrity during operation; Kapton HN and polydimethylsiloxane are the two most common substrates [48]. Typical power outputs are found to range between 2 to 8.75 µW/cm2 [49]. Liu et al. [50] have presented several different designs for wearable devices for harvesting body heat in a range of applications.
Energy storage devices: The integration of energy-harvesting and storage devices has been extensively investigated for emerging self-powered electronic devices [51]. Thermoelectric generators can be used to convert excess heat generated during the operation of electronic devices into electricity [52]. Using the Soret effect, ionic thermoelectric supercapacitors utilize ionic electrolytes to produce charges and energy storage in a single device; the operation of these devices is, however, limited by long charging and discharging times and a rigid configuration [53][54]. Yang et al. [55] have reported on a TEG device with n- and p- type modules consisting of Ag2Te- and Ag2Se-nanoparticle thin films. This device was directly linked with a planar micro supercapacitor. A Seebeck voltage of 82 mV was generated for ΔT of 15.8 K and a charging efficiency of 98%.
Park et al. [56] have reported an all-in-one energy system consisting of a TEG on one side of the substrate and a micro supercapacitor (MSC) on the other side. The TEG was constructed from screen-printed p- and n- modules and a p- type TE film for alignment with electrodes. An MSC was fabricated on the other side using rGO/CNT electrodes and Au current collectors. Electrodes were positioned above and below the TEG-substrate-MSC system; the electrode on the TEG side was connected externally to the current collector of MSC. This system was able to generate 10.8 V of electrical energy for thermal differences up to 10 K and store it without loss.
5. Conclusions and Future Perspectives
Development of thermoelectrochemical technologies for harvesting waste low-temperature heat opens the prospect of increasing the efficiency of various devices and mechanisms operating in exothermic mode or creating systems for generating electricity based on natural heat sources. Any process that can partially replace fossil fuels as a prime energy source will be used only if it is attractive to industry; an alternative energy source such as waste heat conversion (WHC) has to cost effective to becoming commercially viable. Geoffroy et al. [57] have shown that current WHC heat engines are not economically viable below 100ºC and require temperatures above 150ºC coupled with 100-1000 kW power outputs to be economically competitive. Studies in recent years have shown the possibility of significant increases in the power and conversion efficiency of TEC cells. Highest values of output power and cell potentials have been achieved for the redox ferri/ferrocyanide system and Co2+/3+, which offers great opportunities for further development and research in both aqueous and non-aqueous solvents.
Achieved results show the pathways to overcome key fundamental limitations of thermocell performance and set new tasks for fundamental research and further development of electrodes, electrolyte materials and cell design. One of the key tasks in thermocells development is to investigate mechanisms of entropy change via new redox couples and electrolytes. This will be related to increasing the hypothetical Seebeck coefficient, as well as improving the properties of electrodes and solvents to increase the mass transfer rate (diffusion capacity) towards increasing exchange currents and output power values.
One of the most promising thermoelectric power generation application involves vehicle waste heat recovery to improve fuel economy, wherein waste heat from the exhaust, is redirected to produce electricity. Other applications include harvesting industrial waste heat (incinerators, cement, steel mills etc.), geothermal, fuel oil-fired furnaces or gas water-heaters with this technology. Despite extensive research, WHC technology has yet to achieve significant market penetration. For WHCs to become be a serious contender, it has to compete with solar, wind, geothermal technologies in terms of capacity factors, capital costs, operational as well as maintenance costs.
This entry is adapted from the peer-reviewed paper 10.3390/su14159483
References
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579.
- Straub, A.P.; Yip, N.Y.; Lin, S.; Lee, J.; Elimelech, M. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nat. Energy 2016, 1, 16090.
- Yang, Y.; Lee, S.W.; Ghasemi, H.; Loomis, J.; Li, X.; Kraemer, D.; Zheng, G.; Cui, Y.; Chen, G. Charging-free electrochemical system for harvesting low-grade thermal energy. Proc. Natl. Acad. Sci. USA 2014, 111, 17011–17016.
- Evans, S. CarbonBrief/Energy. Solar, Wind and Nuclear Have ‘Amazingly Low’ Carbon Footprints. 2017. Available online: https://www.carbonbrief.org/solar-wind-nuclear-amazingly-low-carbon-footprints/#:~:text=Simon%20Evans,-08.12.2017%20%7C%205&text=Building%20solar%2C%20wind%20or%20nuclear,of%20electricity%20out%20to%202050 (accessed on 10 January 2022).
- Bouty, E. Phénomènes thermo-électriques et électro-thermiques au contact d’un métal et d’un liquide. J. Phys. Théorique Appliquée 1880, 9, 306–320.
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; Macmillan Publishers, Ltd.: London, UK; World Scientific Publishing Co., Ltd.: Singapore, 2010; pp. 101–110.
- Jangonda, C.; Patil, K.; Kinikar, A.; Bhokare, R.; Gavali, M.D. Review of various application of thermoelectric module. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 3393.
- de Bethune, A.J.; Licht, T.S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. J. Electrochem. Soc. 1959, 106, 616.
- Landry, B.A. Utilization of waste heat. Science 1953, 3, 3.
- Wakao, N.; Nojo, K. Nitric acid cycle process for extracting thermal energy from low-level heat sources. Nature 1978, 273, 25–27.
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602.
- Waske, A.; Dzekan, D.; Sellschopp, K.; Berger, D.; Stork, A.; Nielsch, K.; Fähler, S. Energy harvesting near room temperature using a thermomagnetic generator with a pretzel-like magnetic flux topology. Nat. Energy 2018, 4, 68–74.
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613.
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z.L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346.
- Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L.W. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nat. Mater. 2018, 17, 432–438.
- Thakre, A.; Kumar, A.; Song, H.C.; Jeong, D.Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, 19, 2170.
- Quoilin, S.; van den Broek, M.; Declaye, S.; Dewallef, P.; Lemort, V. Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renew. Sustain. Energy Rev. 2013, 22, 168–186.
- Zhang, X.; Chau, K.T. An automotive thermoelectric photovoltaic hybrid energy system using maximum power point tracking. Energy Convers. Manag. 2011, 52, 641e7.
- Zhang, H.; W Kong, W.; Dong, F.; Xu, H.; Chen, B.; Ni, M. Application of cascading thermoelectric generator and cooler for waste heat recovery from solid oxide fuel cells. Energy Convers. Manag. 2017, 148, 1382–1390.
- Li, P.; Cai, L.; Zhai, P.; Tang, X.; Zhang, Q.; Nino, M. Design of a concentration solar thermoelectric generator. J. Electron. Mater. 2010, 39, 1522–1530.
- Dupont, M.F.; MacFarlane, D.R.; Pringle, J.M. Thermo-electrochemical cells for waste heat harvesting—Progress and perspectives. Chem. Commun. 2017, 53, 6288–6302.
- Lalancette, J.-M.; Roussel, R. Metals intercalated in graphite. V. A concentration cell with intercalated bromine. Can. J. Chem. 1976, 54, 3541–3544.
- Endo, M.; Yamagishi, Y.; Inagaki, M. Thermocell with graphite fiber-bromine intercalation compounds. Synth. Met. 1983, 7, 203–209.
- Inagaki, M.; Matsumoto, A.; Sakai, M.; Maeda, Y. A cell of carbon-fibers and nitric acid with temperature difference. Nippon Kagaku Kaishi 1983, 2, 309–311.
- Maeda, Y.; Kitamura, H.; Itoh, E.; Inagaki, M. A new carbon fiber and nitric acid cell with a temperature difference between electrodes. Synth. Met. 1983, 7, 211–217.
- Mua, Y.; Quickenden, T.I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. J. Electrochem. Soc. 1996, 143, 2558–2564.
- Wang, W.; Shu, G.; Tian, H.; Huo, D.; Zhu, X. A bimetallic thermally-regenerative ammonia-based flow battery for low-grade waste heat recovery. J. Power Sources 2019, 424, 184–192.
- Inagaki, M.; Itoh, E.; Maeda, Y. Durable Performance of Thermocell with Carbon Cloth and Nitric Acid. TANSO 1985, 1985, 134–136.
- Cheng, C.; Dai, Y.; Yu, J.; Liu, C.; Wang, S.; Feng, S.P.; Ni, M. Review of Liquid-Based Systems to Recover Low-Grade Waste Heat for Electrical Energy Generation. Energy Fuels 2021, 35, 161–175.
- Hu, R.; Xu, D.; Luo, X. Liquid Thermocells Enable Low-Grade Heat Harvesting. Matter 2020, 3, 1400–1402.
- Black, J.J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Phys. Chem. Chem. Phys. 2016, 18, 20768–20777.
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Appl. Energy Mater. 2018, 1, 1424–1428.
- Burmistrov, I.; Artyukhov, D.; Shindrov, A.; Gorshkov, N.; Gorokhovsky, A. Thermo-Electrochemical Cells for Low-Grade Waste Heat Conversion. In Proceedings of the Nanotech Middle East 2017 Conference and Exhibition, Dubai, United Arab Emirates, 4–6 December 2017; pp. 4–6.
- Quickenden, T.I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. J. Electrochem. Soc. 1995, 142, 3985–3994.
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779.
- Gunawan, A.; Li, H.; Lin, C.H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. The amplifying effect of natural convection on power generation of thermogalvanic cells. Int. J. Heat Mass Transf. 2014, 78, 423–434.
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renew. Energy Focus 2019, 29, 42–48.
- Koo, M.H.; Yoon, H.H. Fabrication of carbon nanotubes and charge transfer complex-based electrodes for a glucose/oxygen biofuel cell. J. Nanosci. Nanotechnol. 2013, 13, 7434–7438.
- Nugent, J.M.; Santhanam, K.S.V.; Rubio, A.; Ajayan, P.M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Lett. 2001, 1, 87–91.
- Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E.B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renew. Energy 2020, 157, 1–8.
- Kiselev, N.; Artyukhov, D.; Boychenko, E.; Gorshkov, N.; Glubokaya, A.; Burmistrov, I. Electrolyte concentration dependences of NiO based thermoelectrochemical cells performance. AIP Conf. Proc. 2022, 2456, 020005.
- Taganova, A.A.; Boychenko, E.A.; Kiselev, N.v.; Khaidarov, B.B.; Kolesnikov, E.A.; Yudin, A.G.; Vikulova, M.A.; Gorshkov, N.v.; Kuznetsov, D.v.; Burmistrov, I.N. Synthesis and Study of the Composition of Hollow Microspheres of NiO and NiO/Ni Composition for Thermoelectrochemical Energy Converters of Low-Potential Temperature Gradients of Thermal Units into Electricity. Refract. Ind. Ceram. 2021, 61, 715–719.
- Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.; Lach, J.; Lee, B.; Muth, J.; Oralkan, O.; Ozturk, M.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proc. IEEE 2015, 103, 665–681.
- Ando Junior, O.H.; Maran, A.L.O.; Henao, N.C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renew. Sustain. Energy Rev. 2018, 91, 376–393.
- Riemer, R.; Shapiro, A. Biomechanical energy harvesting from human motion: Theory, state of the art, design guidelines, and future directions. J. Neuroeng. Rehabil. 2011, 8, 22.
- Invernizzi, F.; Dulio, S.; Patrini, M.; Guizzetti, G.; Mustarelli, P. Energy harvesting from human motion: Materials and techniques. Chem. Soc. Rev. 2016, 45, 5455–5473.
- Llamas, R.; IDC Media Center. Worldwide Wearables Market to Nearly Double by 2021, According to IDC Internet. Available online: https://www.idc.com/getdoc.jsp?containerId=prUS42818517 (accessed on 22 November 2021).
- Park, H.; Lee, D.; Kim, D.; Cho, H.; Eom, Y.; Hwang, J.; Kim, H.; Kim, J.; Han, S.; Kim, W. High power output from body heat harvesting based on flexible thermoelectric system with low thermal contact resistance. J. Phys. D Appl. Phys. 2018, 51, 365501.
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Adv. Electron. Mater. 2019, 5, 1800769.
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669.
- Guan, X.; Cheng, H.; Ouyang, J. Significant enhancement in the Seebeck coefficient and power factor of thermoelectric polymers by the Soret effect of polyelectrolytes. J. Mater. Chem. A 2018, 6, 19347–19352.
- Yang, S.; Cho, K.; Park, Y.; Kim, S. Bendable thermoelectric generators composed of p- and n-type silver chalcogenide nanoparticle thin films. Nano Energy 2018, 49, 333–337.
- Kim, S.J.; Lee, H.E.; Choi, H.; Kim, Y.; We, J.H.; Shin, J.S.; Lee, K.J.; Cho, B.J. High-Performance Flexible Thermoelectric Power Generator Using Laser Multiscanning Lift-Off Process. ACS Nano 2016, 10, 10851–10857.
- Zhao, D.; Wang, H.; Khan, Z.U.; Chen, J.C.; Gabrielsson, R.; Jonsson, M.P.; Berggren, M.; Crispin, X. Ionic thermoelectric supercapacitors. Energy Environ. Sci. 2016, 9, 1450–1457.
- Yang, K.; Cho, K.; Yang, S.; Park, Y.; Kim, S. A laterally designed all-in-one energy device using a thermoelectric generator-coupled micro supercapacitor. Nano Energy 2019, 60, 667–672.
- Park, Y.; Cho, K.; Kim, S. Vertical all-in-one energy systems constructed with thermoelectric generators and microsupercapacitors. J. Power Sources 2021, 510, 230402.
- Geffroy, C.; Lilley, D.; Parez, P.S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096.
