Tumor necrosis factor (TNF) receptor associated factor-2 (TRAF2) is an intracellular adapter protein with E3 ligase activity, which interacts with a plethora of other signaling proteins, including plasma membrane receptors, kinases, phosphatases, other E3 ligases, and deubiquitinases. TRAF2 is involved in various cancer-relevant cellular processes, such as the activation of transcription factors of the NFκB family, stimulation of mitogen-activated protein (MAP) kinase cascades, endoplasmic reticulum (ER) stress signaling, autophagy, and the control of cell death programs. In a context-dependent manner, TRAF2 promotes tumor development but it can also act as a tumor suppressor.
- apoptosis
- autophagy
- B-cell lymphoma
- cellular inhibitor of apoptosis 1/2 (cIAP1/2)
- necroptosis
1. Introduction
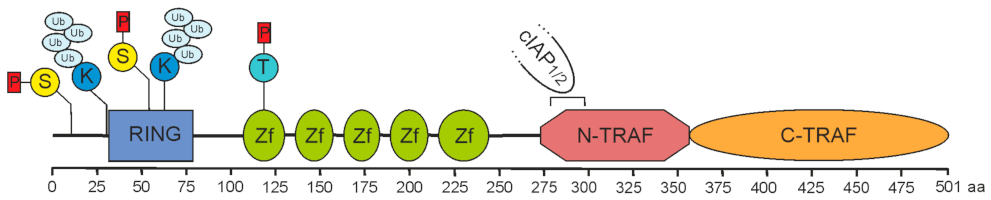
| Protein | Type of Protein | Experimental Evidence |
Target Domain in TRAF | Reference |
|---|---|---|---|---|
| TNFR2 | TNFRSF | THS, GST, endo Co-IP | CTD | [2] |
| LTβR | TNFRSF | endo Co-IP | [11] | |
| OX40 | TNFRSF | THS, Co-IP | [12][13] | |
| CD40 | TNFRSF | THS, GST, endo Co-IP | [14] | |
| CD27 | TNFRSF | THS, Co-IP | [15][16][17] | |
| CD30 | TNFRSF | THS, GST | [18][19] | |
| 4-1BB | TNFRSF | THS, GST, endo Co-IP | [12][20][21] | |
| RANK | TNFRSF | GST, Co-IP | [22][23][24] | |
| Fn14 | TNFRSF | GST | [25] | |
| TACI | TNFRSF | THS, Co-IP | [26] | |
| HVEM | TNFRSF | GST | [27] | |
| NGFR | TNFRSF | Co-IP | [28] | |
| BCMA | TNFRSF | Co-IP | [29] | |
| GITR | TNFRSF | THS, endo Co-IP | [30][31] | |
| TROY | TNFRSF | Co-IP | [32] | |
| IL15R | receptor | Co-IP | [33] | |
| IFNαR1 | receptor | GST, Co-IP | [34] | |
| LMP1 | viral oncogen | GST, Co-IP | [35][36] | |
| A20 | DUB, E3 ligase | THS, Co-IP | [37] | |
| AIP1 | Ras-GAP | Co-IP | RING/zinc | [38] |
| AIMP2 | adaptor | THS, GST, endo Co-IP | [39] | |
| APPL1 | adaptor | GST, endo Co-IP | [40] | |
| AWP1 | adaptor | THS, Co-IP | TD | [41] |
| Bcl10 | adaptor | THS, Co-IP | [42] | |
| Beclin | autophagy | GST, endo Co-IP | RING | [43] |
| Caspase-2 | caspase | Endo Co-IP | [44] | |
| Caspase-12 | caspase | Co-IP | NTD | [45] |
| β-catenin | proto-oncogene | Co-IP, MST | TD | [46] |
| caveolin-1 | plasma membrane protein | endo Co-IP | [47] | |
| CHIP | E3 ligase | endo Co-IP | [48] | |
| cIAP1 | E3 ligase | THS, Co-IP | NTD | [1] |
| cIAP2 | E3 ligase | THS, Co-IP | NTD | [1] |
| CYLD | DUB | THS, Co-IP | TD | [49] |
| DUSP14 | phosphatase | Co-IP | [50] | |
| DYRK1A | kinase | endo Co-IP | TD | [51] |
| EGFR | kinase | endo Co-IP | [52] | |
| EI24 | E3 ligase | Co-IP | [53] | |
| eIF4GI | scaffold | THS, GST, Co-IP | TD | [54] |
| Eva1 | adhesion protein | endo Co-IP | [55] | |
| FAK | kinase | endo Co-IP | [56] | |
| Filamin | actin binder | Co-IP | RZ | [57] |
| GCKR | kinase | endo Co-IP | TD | [58] |
| Gpx1 | peroxidase | Co-IP | TD | [59] |
| GRA15 | virulence factor | Co-IP | [60] | |
| GSTP1-1 | gluthation transferase | Co-IP | [61] | |
| HGK | Kinase | Co-IP | [62] | |
| Hoxa1 | transcription factor | THS, Co-IP | [63] | |
| HSP70 | Chaperon | Co-IP | TD | [64] |
| IKK1 | kinase | GST, endo Co-IP | RING | [65] |
| IKK2 | kinase | GST, endo Co-IP | RING | [65] |
| IKKe | kinase | Co-IP | [66] | |
| IRE1 | kinase, nuclease | endo Co-IP | [67] | |
| I-TRAF | adaptor | THS, GST, Co-IP | TD | [68] |
| JIK | kinase | Co-IP | [45] | |
| KRC | DNA binding | endo Co-IP | TD | [69] |
| LGP2 | RLR | Co-IP | CTD | [70] |
| LILRB3 | receptor | endo Co-IP | [71] | |
| LRPPRC | RNA regulation | Co-IP | [72] | |
| MAVS | adaptor | Co-IP | CTD | [73][74] |
| MEKK1 | kinase | Co-IP | [75] | |
| MIZ | transcription factor | GST, endo Co-IP | RING | [76] |
| MLK3 | kinase | Co-IP | NTD | [77][78] |
| MST1 | kinase | endo Co-IP | Zn fingers | [79] |
| TRIM37 | E3 ligase | Co-IP | TD | [80] |
| Nef | virulence factor | GST | [81][82] | |
| HCV core | virulence factor | GST | [81] | |
| NIP45 | transcription factor associated | endo Co-IP | [83] | |
| NSP1 | virulence factor | Co-IP | [84] | |
| Nur77 | nuclear receptor | Co-IP | RING, NTD | [85] |
| parkin | E3 ligase | endo Co-IP | [86] | |
| proPTPRN2 | phosphatase | Co-IP | RING | [87] |
| RET/PTC3 | oncogenic RTK fusion protein | Co-IP | [88] | |
| RIPK1 | kinase | Co-IP | NTD, CTD | [89] |
| RIP2 | kinase | Co-IP | [90] | |
| RNAseT2 | ribonuclease | Co-IP | [91] | |
| RSK2 | kinase | Co-IP | [92] | |
| SHP-1 | phosphatase | Co-IP | [93] | |
| SGEF | GEF | Co-IP | TD | [94] |
| Sharpin | scaffold | Co-IP | [95] | |
| SIAH-2 | E3 ligase | GST | [96] | |
| SMAD4 | signaling protein | THS, endo Co-IP | [97] | |
| SMURF-2 | E3 ligase | THS, Co-IP | [98] | |
| SMYD2 | methyltransferase | Co-IP, SPR | [99] | |
| SphK1 | kinase | GST, Co-IP | [100] | |
| T2BP / TIFA | adaptor | THS, Co-IP | TD | [101] |
| TCPTP | phosphatase | endo Co-IP | [102] | |
| TPL2/COT1 | kinase | Co-IP | [103] | |
| TAK1 | kinase | Co-IP | [104] | |
| TBK1 | kinase | endo Co-IP | NTD | [74] |
| TNIK | kinase | Co-IP | TD | [105] |
| TRADD | adaptor | THS, Co-IP | CTD | [89][106] |
| TRAF1 | adaptor | THS, Co-IP | NTD, CTD | [2][89] |
| TRAF2 | E3 ligase, adaptor | THS, Co-IP | CTD | [2][89] |
| TRAF3 | E3 ligase, adaptor | Co-IP | [107] | |
| TRAF4 | E3 ligase, adaptor | endo Co-IP | [108] | |
| TRIF | adaptor | THS, Co-IP | [109] | |
| UBC13 | E2 | endo Co-IP | RING | [76] |
| USP4 | DUB | Co-IP | [110] | |
| USP7 | DUB | GST | [80] | |
| USP17 | DUB | Co-IP | [107] | |
| UXT-V1 | transcriptional cofactor | endo Co-IP | [111] | |
| VP4 | rotavirus capsid protein | THS, Co-IP | [112] | |
| WDR62 | scaffold | Co-IP | [113] |
2. Role of TRAF2 in Immune Signaling Pathways
2.1. TRAF2 and Activation of the Classical NFκB Pathway
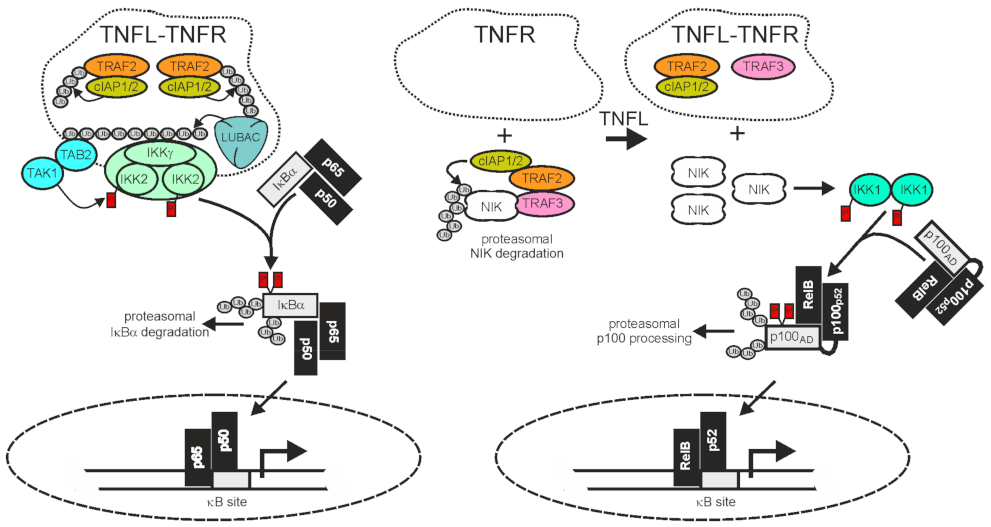
2.2. TRAF2 and Activation of the Alternative NFκB Pathway
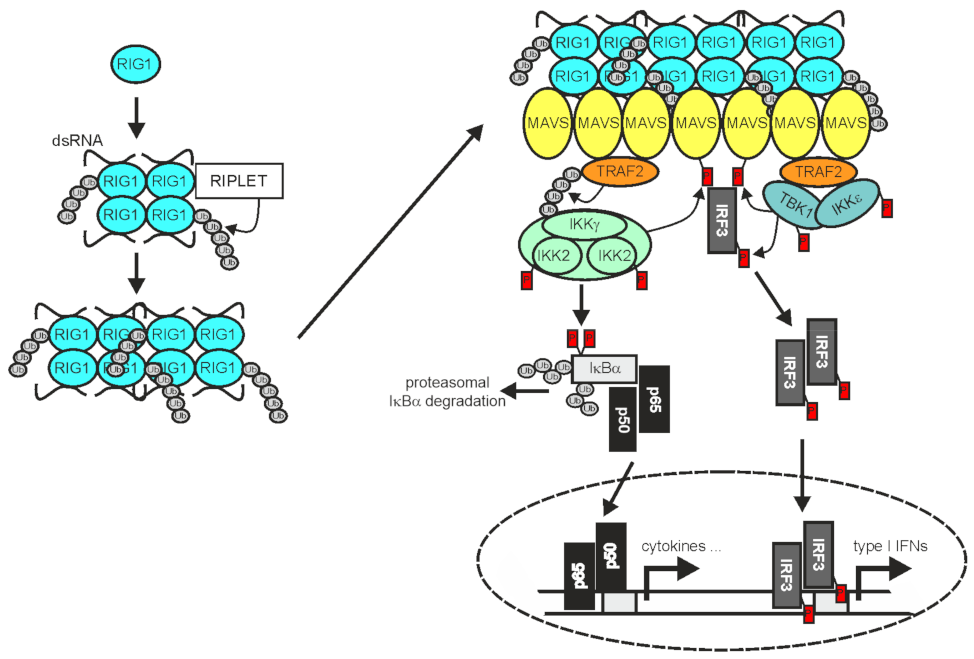
2.3. TRAF2 in RLR Signaling
This entry is adapted from the peer-reviewed paper 10.3390/cancers14164055
References
- Rothe, M.; Pan, M.G.; Henzel, W.J.; Ayres, T.M.; Goeddel, D.V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995, 83, 1243–1252.
- Rothe, M.; Wong, S.C.; Henzel, W.J.; Goeddel, D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 1994, 78, 681–692.
- Wajant, H.; Grell, M.; Scheurich, P. TNF receptor associated factors in cytokine signaling. Cytokine Growth Factor Rev. 1999, 10, 15–26.
- Vince, J.E.; Pantaki, D.; Feltham, R.; Mace, P.D.; Cordier, S.M.; Schmukle, A.C.; Davidson, A.J.; Callus, B.A.; Wong, W.W.; Gentle, I.E.; et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-b and to prevent tnf-induced apoptosis. J. Biol. Chem. 2009, 284, 35906–35915.
- Workman, L.M.; Zhang, L.; Fan, Y.; Zhang, W.; Habelhah, H. TRAF2 Ser-11 Phosphorylation Promotes Cytosolic Translocation of the CD40 Complex To Regulate Downstream Signaling Pathways. Mol. Cell Biol. 2020, 40, e00429-19.
- Zhou, W.; Yao, J.; Wang, G.; Chen, Z.; Li, Z.; Feng, D.; Li, Y.; Qasim, W.; Tan, W.; Ning, S.; et al. PKCζ phosphorylates TRAF2 to protect against intestinal ischemia-reperfusion-induced injury. Cell Death Dis. 2017, 8, e2935.
- Thomas, G.S.; Zhang, L.; Blackwell, K.; Habelhah, H. Phosphorylation of TRAF2 within its RING domain inhibits stress-induced cell death by promoting IKK and suppressing JNK activation. Cancer Res. 2009, 69, 3665–3672.
- Qian, Y.; Wang, Z.; Lin, H.; Lei, T.; Zhou, Z.; Huang, W.; Wu, X.; Zuo, L.; Wu, J.; Liu, Y.; et al. TRIM47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via K63-linked ubiquitination of TRAF2. Signal. Transduct. Target. Ther. 2022, 7, 148.
- Li, S.; Wang, L.; Dorf, M.E. PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination. Mol. Cell 2009, 33, 30–42.
- Li, S.; Wang, L.; Berman, M.A.; Zhang, Y.; Dorf, M.E. RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling. Mol. Cell 2006, 24, 497–509.
- Kim, Y.S.; Nedospasov, S.A.; Liu, Z.G. TRAF2 plays a key, nonredundant role in LIGHT-lymphotoxin beta receptor signaling. Mol. Cell Biol. 2005, 25, 2130–2137.
- Arch, R.H.; Thompson, C.B. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol. Cell Biol. 1998, 18, 558–565.
- Kawamata, S.; Hori, T.; Imura, A.; Takaori-Kondo, A.; Uchiyama, T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998, 273, 5808–5814.
- Rothe, M.; Sarma, V.; Dixit, V.M.; Goeddel, D.V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 1995, 269, 1424–1427.
- Akiba, H.; Nakano, H.; Nishinaka, S.; Shindo, M.; Kobata, T.; Atsuta, M.; Morimoto, C.; Ware, C.F.; Malinin, N.L.; Wallach, D.; et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J. Biol. Chem. 1998, 273, 13353–13358.
- Gravestein, L.A.; Amsen, D.; Boes, M.; Calvo, C.R.; Kruisbeek, A.M.; Borst, J. The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. Eur J. Immunol. 1998, 28, 2208–2216.
- Yamamoto, H.; Kishimoto, T.; Minamoto, S. NF-kappaB activation in CD27 signaling: Involvement of TNF receptor-associated factors in its signaling and identification of functional region of CD27. J. Immunol. 1998, 161, 4753–4759.
- Gedrich, R.W.; Gilfillan, M.C.; Duckett, C.S.; Van Dongen, J.L.; Thompson, C.B. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J. Biol. Chem. 1996, 271, 12852–12858.
- Lee, S.Y.; Park, C.G.; Choi, Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J. Exp. Med. 1996, 183, 669–674.
- Jang, I.K.; Lee, Z.H.; Kim, Y.J.; Kim, S.H.; Kwon, B.S. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 1998, 242, 613–620.
- Saoulli, K.; Lee, S.Y.; Cannons, J.L.; Yeh, W.C.; Santana, A.; Goldstein, M.D.; Bangia, N.; DeBenedette, M.A.; Mak, T.W.; Choi, Y.; et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 1998, 187, 1849–1862.
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J. Biol. Chem. 1998, 273, 20551–20555.
- Galibert, L.; Tometsko, M.E.; Anderson, D.M.; Cosman, D.; Dougall, W.C. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J. Biol. Chem. 1998, 273, 34120–34127.
- Wong, B.R.; Josien, R.; Lee, S.Y.; Vologodskaia, M.; Steinman, R.M.; Choi, Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 1998, 273, 28355–28359.
- Wiley, S.R.; Cassiano, L.; Lofton, T.; Davis-Smith, T.; Winkles, J.A.; Lindner, V.; Liu, H.; Daniel, T.O.; Smith, C.A.; Fanslow, W.C. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 2001, 15, 837–846.
- Xia, X.Z.; Treanor, J.; Senaldi, G.; Khare, S.D.; Boone, T.; Kelley, M.; Theill, L.E.; Colombero, A.; Solovyev, I.; Lee, F.; et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J. Exp. Med. 2000, 192, 137–143.
- Marsters, S.A.; Ayres, T.M.; Skubatch, M.; Gray, C.L.; Rothe, M.; Ashkenazi, A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1997, 272, 14029–14032.
- Ye, X.; Mehlen, P.; Rabizadeh, S.; VanArsdale, T.; Zhang, H.; Shin, H.; Wang, J.J.; Leo, E.; Zapata, J.; Hauser, C.A.; et al. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J. Biol. Chem. 1999, 274, 30202–30208.
- Hatzoglou, A.; Roussel, J.; Bourgeade, M.F.; Rogier, E.; Madry, C.; Inoue, J.; Devergne, O.; Tsapis, A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000, 165, 1322–1330.
- Esparza, E.M.; Arch, R.H. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappa B activation. J. Immunol. 2005, 174, 7875–7882.
- Kwon, B.; Yu, K.Y.; Ni, J.; Yu, G.L.; Jang, I.K.; Kim, Y.J.; Xing, L.; Liu, D.; Wang, S.X.; Kwon, B.S. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 1999, 274, 6056–6061.
- Eby, M.T.; Jasmin, A.; Kumar, A.; Sharma, K.; Chaudhary, P.M. TAJ, a novel member of the tumor necrosis factor receptor family, activates the c-Jun N-terminal kinase pathway and mediates caspase-independent cell death. J. Biol. Chem. 2000, 275, 15336–15342.
- Pereno, R.; Giron-Michel, J.; Gaggero, A.; Cazes, E.; Meazza, R.; Monetti, M.; Monaco, E.; Mishal, Z.; Jasmin, C.; Indiveri, F.; et al. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene 2000, 19, 5153–5162.
- Yang, C.H.; Murti, A.; Pfeffer, L.M. Interferon induces NF-kappa B-inducing kinase/tumor necrosis factor receptor-associated factor-dependent NF-kappa B activation to promote cell survival. J. Biol. Chem. 2005, 280, 31530–31536.
- Kaye, K.M.; Devergne, O.; Harada, J.N.; Izumi, K.M.; Yalamanchili, R.; Kieff, E.; Mosialos, G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA 1996, 93, 11085–11090.
- Mosialos, G.; Birkenbach, M.; Yalamanchili, R.; VanArsdale, T.; Ware, C.; Kieff, E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 1995, 80, 389–399.
- Song, H.Y.; Rothe, M.; Goeddel, D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc. Natl. Acad. Sci. USA 1996, 93, 6721–6725.
- Zhang, H.; Zhang, R.; Luo, Y.; D’Alessio, A.; Pober, J.S.; Min, W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. J. Biol. Chem. 2004, 279, 44955–44965.
- Choi, J.W.; Kim, D.G.; Park, M.C.; Um, J.Y.; Han, J.M.; Park, S.G.; Choi, E.C.; Kim, S. AIMP2 promotes TNFalpha-dependent apoptosis via ubiquitin-mediated degradation of TRAF2. J. Cell Sci. 2009, 122, 2710–2715.
- Hupalowska, A.; Pyrzynska, B.; Miaczynska, M. APPL1 regulates basal NF-κB activity by stabilizing NIK. J. Cell Sci. 2012, 125, 4090–4102.
- Chang, E.J.; Ha, J.; Kang, S.S.; Lee, Z.H.; Kim, H.H. AWP1 binds to tumor necrosis factor receptor-associated factor 2 (TRAF2) and is involved in TRAF2-mediated nuclear factor-kappaB signaling. Int J. Biochem. Cell Biol. 2011, 43, 1612–1620.
- Yoneda, T.; Imaizumi, K.; Maeda, M.; Yui, D.; Manabe, T.; Katayama, T.; Sato, N.; Gomi, F.; Morihara, T.; Mori, Y.; et al. Regulatory mechanisms of TRAF2-mediated signal transduction by Bcl10, a MALT lymphoma-associated protein. J. Biol. Chem. 2000, 275, 11114–11120.
- Liu, H.; Ma, Y.; He, H.W.; Zhao, W.L.; Shao, R.G. SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy 2017, 13, 900–913.
- Lamkanfi, M.; D’Hondt, K.; Vande Walle, L.; van Gurp, M.; Denecker, G.; Demeulemeester, J.; Kalai, M.; Declercq, W.; Saelens, X.; Vandenabeele, P. A novel caspase-2 complex containing TRAF2 and RIP1. J. Biol. Chem. 2005, 280, 6923–6932.
- Yoneda, T.; Imaizumi, K.; Oono, K.; Yui, D.; Gomi, F.; Katayama, T.; Tohyama, M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 2001, 276, 13935–13940.
- Yan, R.; Zhu, H.; Huang, P.; Yang, M.; Shen, M.; Pan, Y.; Zhang, C.; Zhou, X.; Li, H.; Ke, X.; et al. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022, 38, 110319.
- Feng, X.; Gaeta, M.L.; Madge, L.A.; Yang, J.H.; Bradley, J.R.; Pober, J.S. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J. Biol. Chem. 2001, 276, 8341–8349.
- Jang, K.W.; Lee, K.H.; Kim, S.H.; Jin, T.; Choi, E.Y.; Jeon, H.J.; Kim, E.; Han, Y.S.; Chung, J.H. Ubiquitin ligase CHIP induces TRAF2 proteasomal degradation and NF-κB inactivation to regulate breast cancer cell invasion. J. Cell Biochem. 2011, 112, 3612–3620.
- Kovalenko, A.; Chable-Bessia, C.; Cantarella, G.; Israël, A.; Wallach, D.; Courtois, G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 2003, 424, 801–805.
- Yang, C.Y.; Chiu, L.L.; Tan, T.H. TRAF2-mediated Lys63-linked ubiquitination of DUSP14/MKP6 is essential for its phosphatase activity. Cell Signal. 2016, 28, 145–151.
- Zhang, P.; Zhang, Z.; Fu, Y.; Zhang, Y.; Washburn, M.P.; Florens, L.; Wu, M.; Huang, C.; Hou, Z.; Mohan, M. K63-linked ubiquitination of DYRK1A by TRAF2 alleviates Sprouty 2-mediated degradation of EGFR. Cell Death Dis. 2021, 12, 608.
- Blakely, C.M.; Pazarentzos, E.; Olivas, V.; Asthana, S.; Yan, J.J.; Tan, I.; Hrustanovic, G.; Chan, E.; Lin, L.; Neel, D.S.; et al. NF-κB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015, 11, 98–110.
- Choi, J.M.; Devkota, S.; Sung, Y.H.; Lee, H.W. EI24 regulates epithelial-to-mesenchymal transition and tumor progression by suppressing TRAF2-mediated NF-κB activity. Oncotarget 2013, 4, 2383–2396.
- Kim, W.J.; Back, S.H.; Kim, V.; Ryu, I.; Jang, S.K. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell Biol. 2005, 25, 2450–2462.
- Ohtsu, N.; Nakatani, Y.; Yamashita, D.; Ohue, S.; Ohnishi, T.; Kondo, T. Eva1 Maintains the Stem-like Character of Glioblastoma-Initiating Cells by Activating the Noncanonical NF-κB Signaling Pathway. Cancer Res. 2016, 76, 171–181.
- da Silva, S.D.; Xu, B.; Maschietto, M.; Marchi, F.A.; Alkailani, M.I.; Bijian, K.; Xiao, D.; Alaoui-Jamali, M.A. TRAF2 Cooperates with Focal Adhesion Signaling to Regulate Cancer Cell Susceptibility to Anoikis. Mol. Cancer Ther. 2019, 18, 139–146.
- Leonardi, A.; Ellinger-Ziegelbauer, H.; Franzoso, G.; Brown, K.; Siebenlist, U. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2000, 275, 271–278.
- Shi, C.S.; Leonardi, A.; Kyriakis, J.; Siebenlist, U.; Kehrl, J.H. TNF-mediated activation of the stress-activated protein kinase pathway: TNF receptor-associated factor 2 recruits and activates germinal center kinase related. J. Immunol. 1999, 163, 3279–3285.
- Lee, S.; Lee, E.K.; Kang, D.H.; Lee, J.; Hong, S.H.; Jeong, W.; Kang, S.W. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp. Mol. Med. 2021, 53, 1080–1091.
- Sangaré, L.O.; Yang, N.; Konstantinou, E.K.; Lu, D.; Mukhopadhyay, D.; Young, L.H.; Saeij, J.P.J. Toxoplasma GRA15 Activates the NF-κB Pathway through Interactions with TNF Receptor-Associated Factors. mBio 2019, 10, e00808-19.
- Wu, Y.; Fan, Y.; Xue, B.; Luo, L.; Shen, J.; Zhang, S.; Jiang, Y.; Yin, Z. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 2006, 25, 5787–5800.
- Chuang, H.C.; Sheu, W.H.; Lin, Y.T.; Tsai, C.Y.; Yang, C.Y.; Cheng, Y.J.; Huang, P.Y.; Li, J.P.; Chiu, L.L.; Wang, X.; et al. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat. Commun. 2014, 5, 4602.
- Lambert, B.; Vandeputte, J.; Remacle, S.; Bergiers, I.; Simonis, N.; Twizere, J.C.; Vidal, M.; Rezsohazy, R. Protein interactions of the transcription factor Hoxa1. BMC Dev. Biol. 2012, 12, 29.
- Dai, S.; Jiang, L.; Wang, G.; Zhou, X.; Wei, X.; Cheng, H.; Wu, Z.; Wei, D. HSP70 interacts with TRAF2 and differentially regulates TNFalpha signalling in human colon cancer cells. J. Cell Mol. Med. 2010, 14, 710–725.
- Devin, A.; Lin, Y.; Yamaoka, S.; Li, Z.; Karin, M.; Liu, Z. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol. Cell Biol. 2001, 21, 3986–3994.
- Zhou, A.Y.; Shen, R.R.; Kim, E.; Lock, Y.J.; Xu, M.; Chen, Z.J.; Hahn, W.C. IKKε-mediated tumorigenesis requires K63-linked polyubiquitination by a cIAP1/cIAP2/TRAF2 E3 ubiquitin ligase complex. Cell Rep. 2013, 3, 724–733.
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666.
- Rothe, M.; Xiong, J.; Shu, H.B.; Williamson, K.; Goddard, A.; Goeddel, D.V. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc. Natl. Acad. Sci. USA 1996, 93, 8241–8246.
- Oukka, M.; Kim, S.T.; Lugo, G.; Sun, J.; Wu, L.C.; Glimcher, L.H. A mammalian homolog of Drosophila schnurri, KRC, regulates TNF receptor-driven responses and interacts with TRAF2. Mol. Cell 2002, 9, 121–131.
- Parisien, J.P.; Lenoir, J.J.; Mandhana, R.; Rodriguez, K.R.; Qian, K.; Bruns, A.M.; Horvath, C.M. RNA sensor LGP2 inhibits TRAF ubiquitin ligase to negatively regulate innate immune signaling. EMBO Rep. 2018, 19, e45176.
- Wu, G.; Xu, Y.; Schultz, R.D.; Chen, H.; Xie, J.; Deng, M.; Liu, X.; Gui, X.; John, S.; Lu, Z.; et al. LILRB3 supports acute myeloid leukemia development and regulates T-cell antitumor immune responses through the TRAF2-cFLIP-NF-κB signaling axis. Nat. Cancer 2021, 2, 1170–1184.
- Jia, H.; Yang, Y.; Li, M.; Chu, Y.; Song, H.; Zhang, J.; Zhang, D.; Zhang, Q.; Xu, Y.; Wang, J.; et al. Snail enhances arginine synthesis by inhibiting ubiquitination-mediated degradation of ASS1. EMBO Rep. 2021, 22, e51780.
- Xu, L.G.; Wang, Y.Y.; Han, K.J.; Li, L.Y.; Zhai, Z.; Shu, H.B. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 2005, 19, 727–740.
- Fang, R.; Jiang, Q.; Zhou, X.; Wang, C.; Guan, Y.; Tao, J.; Xi, J.; Feng, J.M.; Jiang, Z. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 2017, 13, e1006720.
- Baud, V.; Liu, Z.G.; Bennett, B.; Suzuki, N.; Xia, Y.; Karin, M. Signaling by proinflammatory cytokines: Oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999, 13, 1297–1308.
- Liu, J.; Yan, J.; Jiang, S.; Wen, J.; Chen, L.; Zhao, Y.; Lin, A. Site-specific ubiquitination is required for relieving the transcription factor Miz1-mediated suppression on TNF-α-induced JNK activation and inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 191–196.
- Korchnak, A.C.; Zhan, Y.; Aguilar, M.T.; Chadee, D.N. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell. Signal. 2009, 21, 1620–1625.
- Sondarva, G.; Kundu, C.N.; Mehrotra, S.; Mishra, R.; Rangasamy, V.; Sathyanarayana, P.; Ray, R.S.; Rana, B.; Rana, A. TRAF2-MLK3 interaction is essential for TNF-alpha-induced MLK3 activation. Cell Res. 2010, 20, 89–98.
- Roh, K.H.; Choi, E.J. TRAF2 functions as an activator switch in the reactive oxygen species-induced stimulation of MST1. Free Radic. Biol. Med. 2016, 91, 105–113.
- Zapata, J.M.; Pawlowski, K.; Haas, E.; Ware, C.F.; Godzik, A.; Reed, J.C. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J. Biol. Chem. 2001, 276, 24242–24252.
- Khan, K.A.; Abbas, W.; Varin, A.; Kumar, A.; Di Martino, V.; Dichamp, I.; Herbein, G. HIV-1 Nef interacts with HCV Core, recruits TRAF2, TRAF5 and TRAF6, and stimulates HIV-1 replication in macrophages. J. Innate Immun. 2013, 5, 639–656.
- Mangino, G.; Percario, Z.A.; Fiorucci, G.; Vaccari, G.; Acconcia, F.; Chiarabelli, C.; Leone, S.; Noto, A.; Horenkamp, F.A.; Manrique, S.; et al. HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: Involvement of TNF alpha receptor associated factor 2. PLoS ONE 2011, 6, e22982.
- Lieberson, R.; Mowen, K.A.; McBride, K.D.; Leautaud, V.; Zhang, X.; Suh, W.K.; Wu, L.; Glimcher, L.H. Tumor necrosis factor receptor-associated factor (TRAF)2 represses the T helper cell type 2 response through interaction with NFAT-interacting protein (NIP45). J. Exp. Med. 2001, 194, 89–98.
- Bagchi, P.; Bhowmick, R.; Nandi, S.; Kant Nayak, M.; Chawla-Sarkar, M. Rotavirus NSP1 inhibits interferon induced non-canonical NFκB activation by interacting with TNF receptor associated factor 2. Virology 2013, 444, 41–44.
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 2017, 66, 141–153.e146.
- Henn, I.H.; Bouman, L.; Schlehe, J.S.; Schlierf, A.; Schramm, J.E.; Wegener, E.; Nakaso, K.; Culmsee, C.; Berninger, B.; Krappmann, D.; et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J. Neurosci. 2007, 27, 1868–1878.
- Sorokin, A.V.; Nair, B.C.; Wei, Y.; Aziz, K.E.; Evdokimova, V.; Hung, M.C.; Chen, J. Aberrant Expression of proPTPRN2 in Cancer Cells Confers Resistance to Apoptosis. Cancer Res. 2015, 75, 1846–1858.
- Wixted, J.H.; Rothstein, J.L.; Eisenlohr, L.C. Identification of functionally distinct TRAF proinflammatory and phosphatidylinositol 3-kinase/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (PI3K/MEK) transforming activities emanating from RET/PTC fusion oncoprotein. J. Biol. Chem. 2012, 287, 3691–3703.
- Takeuchi, M.; Rothe, M.; Goeddel, D.V. Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J. Biol. Chem. 1996, 271, 19935–19942.
- Thome, M.; Hofmann, K.; Burns, K.; Martinon, F.; Bodmer, J.L.; Mattmann, C.; Tschopp, J. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol. 1998, 8, 885–888.
- Wang, Q.; Jiang, M.; Wu, J.; Ma, Y.; Li, T.; Chen, Q.; Zhang, X.; Xiang, L. Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro. Cell Death Dis. 2014, 5, e1022.
- Peng, C.; Zhu, F.; Wen, W.; Yao, K.; Li, S.; Zykova, T.; Liu, K.; Li, X.; Ma, W.Y.; Bode, A.M.; et al. Tumor necrosis factor receptor-associated factor family protein 2 is a key mediator of the epidermal growth factor-induced ribosomal S6 kinase 2/cAMP-responsive element-binding protein/Fos protein signaling pathway. J. Biol. Chem. 2012, 287, 25881–25892.
- Busca, A.; Konarski, Y.; Gajanayaka, N.; O’Hara, S.; Angel, J.; Kozlowski, M.; Kumar, A. cIAP1/2-TRAF2-SHP-1-Src-MyD88 Complex Regulates Lipopolysaccharide-Induced IL-27 Production through NF-κB Activation in Human Macrophages. J. Immunol. 2018, 200, 1593–1606.
- Fortin Ensign, S.P.; Mathews, I.T.; Eschbacher, J.M.; Loftus, J.C.; Symons, M.H.; Tran, N.L. The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2013, 288, 21887–21897.
- Liang, Y. SHARPIN negatively associates with TRAF2-mediated NFκB activation. PLoS ONE 2011, 6, e21696.
- Habelhah, H.; Frew, I.J.; Laine, A.; Janes, P.W.; Relaix, F.; Sassoon, D.; Bowtell, D.D.; Ronai, Z. Stress-induced decrease in TRAF2 stability is mediated by Siah2. Embo. J. 2002, 21, 5756–5765.
- Shimada, K.; Ikeda, K.; Ito, K. Traf2 interacts with Smad4 and regulates BMP signaling pathway in MC3T3-E1 osteoblasts. Biochem. Biophys. Res. Commun. 2009, 390, 775–779.
- Carpentier, I.; Coornaert, B.; Beyaert, R. Smurf2 is a TRAF2 binding protein that triggers TNF-R2 ubiquitination and TNF-R2-induced JNK activation. Biochem. Biophys. Res. Commun. 2008, 374, 752–757.
- Wu, W.; Wang, J.; Xiao, C.; Su, Z.; Su, H.; Zhong, W.; Mao, J.; Liu, X.; Zhu, Y.Z. SMYD2-mediated TRAF2 methylation promotes the NF-κB signaling pathways in inflammatory diseases. Clin. Transl. Med. 2021, 11, e591.
- Xia, P.; Wang, L.; Moretti, P.A.; Albanese, N.; Chai, F.; Pitson, S.M.; D’Andrea, R.J.; Gamble, J.R.; Vadas, M.A. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 2002, 277, 7996–8003.
- Kanamori, M.; Suzuki, H.; Saito, R.; Muramatsu, M.; Hayashizaki, Y. T2BP, a novel TRAF2 binding protein, can activate NF-kappaB and AP-1 without TNF stimulation. Biochem. Biophys. Res. Commun. 2002, 290, 1108–1113.
- van Vliet, C.; Bukczynska, P.E.; Puryer, M.A.; Sadek, C.M.; Shields, B.J.; Tremblay, M.L.; Tiganis, T. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 2005, 6, 253–260.
- Eliopoulos, A.G.; Davies, C.; Blake, S.S.; Murray, P.; Najafipour, S.; Tsichlis, P.N.; Young, L.S. The oncogenic protein kinase Tpl-2/Cot contributes to Epstein-Barr virus-encoded latent infection membrane protein 1-induced NF-kappaB signaling downstream of TRAF2. J. Virol. 2002, 76, 4567–4579.
- Hong, S.; Lim, S.; Li, A.G.; Lee, C.; Lee, Y.S.; Lee, E.K.; Park, S.H.; Wang, X.J.; Kim, S.J. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat. Immunol. 2007, 8, 504–513.
- Fu, C.A.; Shen, M.; Huang, B.C.; Lasaga, J.; Payan, D.G.; Luo, Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J. Biol. Chem. 1999, 274, 30729–30737.
- Hsu, H.; Shu, H.B.; Pan, M.G.; Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299–308.
- Lu, C.H.; Yeh, D.W.; Lai, C.Y.; Liu, Y.L.; Huang, L.R.; Lee, A.Y.; Jin, S.C.; Chuang, T.H. USP17 mediates macrophage-promoted inflammation and stemness in lung cancer cells by regulating TRAF2/TRAF3 complex formation. Oncogene 2018, 37, 6327–6340.
- Zhang, X.; Wen, Z.; Sun, L.; Wang, J.; Song, M.; Wang, E.; Mi, X. TRAF2 regulates the cytoplasmic/nuclear distribution of TRAF4 and its biological function in breast cancer cells. Biochem. Biophys. Res. Commun. 2013, 436, 344–348.
- Sasai, M.; Tatematsu, M.; Oshiumi, H.; Funami, K.; Matsumoto, M.; Hatakeyama, S.; Seya, T. Direct binding of TRAF2 and TRAF6 to TICAM-1/TRIF adaptor participates in activation of the Toll-like receptor 3/4 pathway. Mol. Immunol. 2010, 47, 1283–1291.
- Xiao, N.; Li, H.; Luo, J.; Wang, R.; Chen, H.; Chen, J.; Wang, P. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem. J. 2012, 441, 979–986.
- Huang, Y.; Chen, L.; Zhou, Y.; Liu, H.; Yang, J.; Liu, Z.; Wang, C. UXT-V1 protects cells against TNF-induced apoptosis through modulating complex II formation. Mol. Biol. Cell 2011, 22, 1389–1397.
- LaMonica, R.; Kocer, S.S.; Nazarova, J.; Dowling, W.; Geimonen, E.; Shaw, R.D.; Mackow, E.R. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J. Biol. Chem. 2001, 276, 19889–19896.
- Prinz, E.; Aviram, S.; Aronheim, A. WDR62 mediates TNFα-dependent JNK activation via TRAF2-MLK3 axis. Mol. Biol. Cell 2018, 29, 2470–2480.
- Gonzalvez, F.; Lawrence, D.; Yang, B.; Yee, S.; Pitti, R.; Marsters, S.; Pham, V.C.; Stephan, J.P.; Lill, J.; Ashkenazi, A. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol. Cell 2012, 48, 888–899.
- Xu, L.; Zhang, Y.; Qu, X.; Che, X.; Guo, T.; Li, C.; Ma, R.; Fan, Y.; Ma, Y.; Hou, K.; et al. DR5-Cbl-b/c-Cbl-TRAF2 complex inhibits TRAIL-induced apoptosis by promoting TRAF2-mediated polyubiquitination of caspase-8 in gastric cancer cells. Mol. Oncol. 2017, 11, 1733–1751.
- Jin, J.; Xiao, Y.; Hu, H.; Zou, Q.; Li, Y.; Gao, Y.; Ge, W.; Cheng, X.; Sun, S.C. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat. Commun. 2015, 6, 5930.
- Shen, Y.; Liu, W.W.; Zhang, X.; Shi, J.G.; Jiang, S.; Zheng, L.; Qin, Y.; Liu, B.; Shi, J.H. TRAF3 promotes ROS production and pyroptosis by targeting ULK1 ubiquitination in macrophages. Faseb. J. 2020, 34, 7144–7159.
- Yang, K.C.; Ma, X.; Liu, H.; Murphy, J.; Barger, P.M.; Mann, D.L.; Diwan, A. Tumor necrosis factor receptor-associated factor 2 mediates mitochondrial autophagy. Circ. Heart Fail. 2015, 8, 175–187.
- Zachari, M.; Gudmundsson, S.R.; Li, Z.; Manifava, M.; Cugliandolo, F.; Shah, R.; Smith, M.; Stronge, J.; Karanasios, E.; Piunti, C.; et al. Selective Autophagy of Mitochondria on a Ubiquitin-Endoplasmic-Reticulum Platform. Dev. Cell 2019, 50, 627–643.e625.
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558.
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57.
- Brummelkamp, T.R.; Nijman, S.M.; Dirac, A.M.; Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 2003, 424, 797–801.
- Tesio, M.; Tang, Y.; Müdder, K.; Saini, M.; von Paleske, L.; Macintyre, E.; Pasparakis, M.; Waisman, A.; Trumpp, A. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J. Exp. Med. 2015, 212, 525–538.
- Trompouki, E.; Hatzivassiliou, E.; Tsichritzis, T.; Farmer, H.; Ashworth, A.; Mosialos, G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 2003, 424, 793–796.
- Lork, M.; Verhelst, K.; Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: So similar, yet so different. Cell Death Differ. 2017, 24, 1172–1183.
- Borghi, A.; Haegman, M.; Fischer, R.; Carpentier, I.; Bertrand, M.J.M.; Libert, C.; Afonina, I.S.; Beyaert, R. The E3 ubiquitin ligases HOIP and cIAP1 are recruited to the TNFR2 signaling complex and mediate TNFR2-induced canonical NF-κB signaling. Biochem. Pharmacol. 2018, 153, 292–298.
- Lafont, E.; Kantari-Mimoun, C.; Draber, P.; De Miguel, D.; Hartwig, T.; Reichert, M.; Kupka, S.; Shimizu, Y.; Taraborrelli, L.; Spit, M.; et al. The linear ubiquitin chain assembly complex regulates TRAIL-induced gene activation and cell death. Embo. J. 2017, 36, 1147–1166.
- Tokunaga, F.; Nakagawa, T.; Nakahara, M.; Saeki, Y.; Taniguchi, M.; Sakata, S.; Tanaka, K.; Nakano, H.; Iwai, K. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature 2011, 471, 633–636.
- Varfolomeev, E.; Goncharov, T.; Maecker, H.; Zobel, K.; Kömüves, L.G.; Deshayes, K.; Vucic, D. Cellular inhibitors of apoptosis are global regulators of NF-κB and MAPK activation by members of the TNF family of receptors. Sci. Signal. 2012, 5, ra22.
- Xie, P.; Hostager, B.S.; Munroe, M.E.; Moore, C.R.; Bishop, G.A. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J. Immunol. 2006, 176, 5388–5400.
- Rowland, S.L.; Tremblay, M.M.; Ellison, J.M.; Stunz, L.L.; Bishop, G.A.; Hostager, B.S. A novel mechanism for TNFR-associated factor 6-dependent CD40 signaling. J. Immunol. 2007, 179, 4645–4653.
- Vallabhapurapu, S.; Matsuzawa, A.; Zhang, W.; Tseng, P.H.; Keats, J.J.; Wang, H.; Vignali, D.A.; Bergsagel, P.L.; Karin, M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 2008, 9, 1364–1370.
- Zarnegar, B.J.; Wang, Y.; Mahoney, D.J.; Dempsey, P.W.; Cheung, H.H.; He, J.; Shiba, T.; Yang, X.; Yeh, W.C.; Mak, T.W.; et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 2008, 9, 1371–1378.
- Fotin-Mleczek, M.; Henkler, F.; Samel, D.; Reichwein, M.; Hausser, A.; Parmryd, I.; Scheurich, P.; Schmid, J.A.; Wajant, H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002, 115, 2757–2770.
- Vince, J.E.; Chau, D.; Callus, B.; Wong, W.W.; Hawkins, C.J.; Schneider, P.; McKinlay, M.; Benetatos, C.A.; Condon, S.M.; Chunduru, S.K.; et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J. Cell Biol. 2008, 182, 171–184.
- Ablasser, A.; Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020, 21, 17–29.
- Cadena, C.; Ahmad, S.; Xavier, A.; Willemsen, J.; Park, S.; Park, J.W.; Oh, S.W.; Fujita, T.; Hou, F.; Binder, M.; et al. Ubiquitin-Dependent and -Independent Roles of E3 Ligase RIPLET in Innate Immunity. Cell 2019, 177, 1187–1200.e1116.
- Jiang, X.; Kinch, L.N.; Brautigam, C.A.; Chen, X.; Du, F.; Grishin, N.V.; Chen, Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 2012, 36, 959–973.
- Peisley, A.; Wu, B.; Yao, H.; Walz, T.; Hur, S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell 2013, 51, 573–583.
- Wu, B.; Peisley, A.; Tetrault, D.; Li, Z.; Egelman, E.H.; Magor, K.E.; Walz, T.; Penczek, P.A.; Hur, S. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol. Cell 2014, 55, 511–523.
- Liu, S.; Chen, J.; Cai, X.; Wu, J.; Chen, X.; Wu, Y.T.; Sun, L.; Chen, Z.J. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife 2013, 2, e00785.
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630.
