Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Before entering metamorphosis, the larvae of Drosophila flies expel a transparent glue from their mouth, which solidifies in contact with air within seconds and fixes the animal to a substrate (wood, leaves, fruits, stones, etc.) for several days until the adult emerges. This glue displays interesting adhesive properties, as it can adhere to various substrates with strengths similar to strongly adhesive commercial tapes.
- bioadhesion
- glue
- Drosophila
- fly
- Sgs
1. Introduction
Bioadhesives are materials naturally produced by living organisms that can stick two separate items together and resist their separation [1]. These materials present singular physicochemical properties that have gone through millions of years of evolution. They are of commercial interest because they are made of proteins and sugars and hence are safe for the human body and the environment. Dental, medical and industrial applications often require adhesion in wet environments, and the marine mussel’s byssus has become a leading model for biomimetic wet adhesion [2][3]. Still, mussel-inspired bioadhesives are so far only used in research and have not been tested through clinical trials [2]. They have been used as a model to perform sutureless wound closure or to seal a fetal membrane. These glue components may also have anticancer and antimicrobial applications thanks to their sticky properties at the cellular level that enable them to target cancer or microbe cells. They also possess antifouling properties that can be used to control absorption of cells or proteins into a surface [3].
In contrast, bioadhesives that work in dry environments are less well characterized. The glue produced by Drosophila flies to stick themselves to a substrate for several days during metamorphosis appears to be a promising model for biomimetic dry adhesion. This glue is produced by the animal at the third instar larval wandering stage [4], a developmental stage during which the Drosophila larva does not feed and is searching for an appropriate site to undergo metamorphosis [5]. The glue is secreted by exocrine cells and accumulates into a pair of salivary glands. Just before entering into metamorphosis, the larva expectorates the entire content of the glands within a minute and the glue is spread all over the body. The glue solidifies rapidly and forms a transparent dry material located at the interface between the substrate and the animal [4]. After expectoration of the fluid, the larval skin hardens and encloses the now-immobile animal. The process that includes the hardening of the larval skin and the adoption by the animal of a characteristic barrel-like shape is named pupariation [5]. At the end of pupariation, the animal is a prepupa. Between 4 and 6 h after pupariation, the epidermis comes off the puparium cuticle, and a gas bubble appears in the abdomen. Eight hours later, the animal molts and technically becomes a pupa [5]. A few days later, an adult emerges and moves out from the pupal case. The glue allows the animal (as a prepupa and then as a pupa) to remain attached for several days onto its substrate, despite temperature variation, wind, rain and other environmental factors. There has likely been strong evolutionary pressure for firm attachment, as it allows the animal to remain within the environment it chose for metamorphosis, thus increasing its chances of survival.
Given the wide diversity of environmental conditions in which they live and the variety of substrates to which they attach, the numerous fly species that produce glue represent a large source of inspiration for biomimetism. Just within the Drosophila genus, more than 1600 species have been described, and they are widely spread around the world [6], with some species present on all continents while others are specific to an island, tropics or deserts [7]. The glue being located at the interface between the animal and the pupariation substrate, its composition and properties might be adapted to the nature of the pupa’s microhabitat. Experiments in the laboratory have found that different species and strains choose distinct pupariation sites according to humidity, light, temperature, larval density, substrate texture and substrate consistency [5][8]. For example, in laboratory conditions, D. busckii and D. simulans prefer to pupariate on humid surfaces, while D. melanogaster and D. hydei prefer dry substrates [9]. D. simulans, D. yakuba, D. mauritania and D. malerkotliana are found to pupariate in fruits rather than on glass walls, whereas D. melanogaster, D. ananassae, D. virilis, D. novamexicana and D. hydei prefer to pupate on the vial walls [10]. Interestingly D. carcinophila and D. endobranchia have adapted to a humid environment as their pupae attach to the surface of the external mouthparts of land crabs [11][12]. Unfortunately, due to their small size (on the order of 1–2 mm length) and their brown color, which is usually hardly distinguishable from the environmental background, pupae are difficult to spot in nature and there is little information about pupariation sites in the wild for the various Drosophila species. While species with narrow ecological niches are expected to have precisely defined pupariation sites, others appear to stick to a large range of substrates. D. melanogaster pupae have been found adhered to multiple substrates, including the dry parts of various rotten fruits, grape stalks and wood [9][10][13][14]. D. simulans and D. buzzatii pupae have been observed on the dry parts of Opuntia ficus-indica cactus [15]. The invasive species D. suzukii and many Hawaiian Drosophila species often pupariate several centimeters deep in the soil [12][16][17]. The pupariation sites described in the literature might constitute only the most visible locations, while other pupa microhabitats, which are not easily accessible, may not be recorded.
In the 1970s and 1980s, the proteins that make up the Drosophila melanogaster glue were characterized biochemically, and their corresponding salivary gland secretory (Sgs) genes were identified. The glue genes then became a premier model to study the regulation of gene expression, with several ecdysone pulses triggering their expression at defined developmental stages. Such studies were facilitated by the presence of polytene chromosomes in the salivary gland cells [18]. Polytene chromosomes are giant chromosomes visible with classical light microscopy that are made of hundreds of sister chromatids packed together, resulting from multiple rounds of endoreplication. A larva possesses one pair of salivary glands, with about 130 secretory cells per gland in D. melanogaster [19]. Each secretory cell contains about a thousand chromatids for each chromosome, thus allowing the production of large amounts of adhesive glue within a short amount of time [20].
2. Aspect and Ultrastructure of Drosophila Glue
When left on glass slides, D. melanogaster animals usually attach on their ventral side, which presents a relatively flat surface, whereas the lateral and dorsal sides are more curved (Figure 1A–C). The glue forms an oval-shaped patch of solid transparent material of approximately 2 mm in length and 0.5 mm in width located at the surface of contact between the animal and the substrate (Figure 1C) [21]. Due to the overall barrel shape of the pupa, glue thickness varies from 0 μm (at the confocal microscope detection limit, in the middle of the surface of contact) to 20 μm (on the edges of the surface of contact). In addition, the glue can be detected when spread as a thin layer of about 0.1 μm onto the substrate outside of the surface of contact, and it also covers the surface of the pupal case that is not in contact with the substrate. It is thus reasonable to assume that the glue has good wetting properties both on the pupa and natural substrates, which probably means that the glue is highly hydrophilic. Further investigation on wetting properties and contact angle measurements will be valuable.
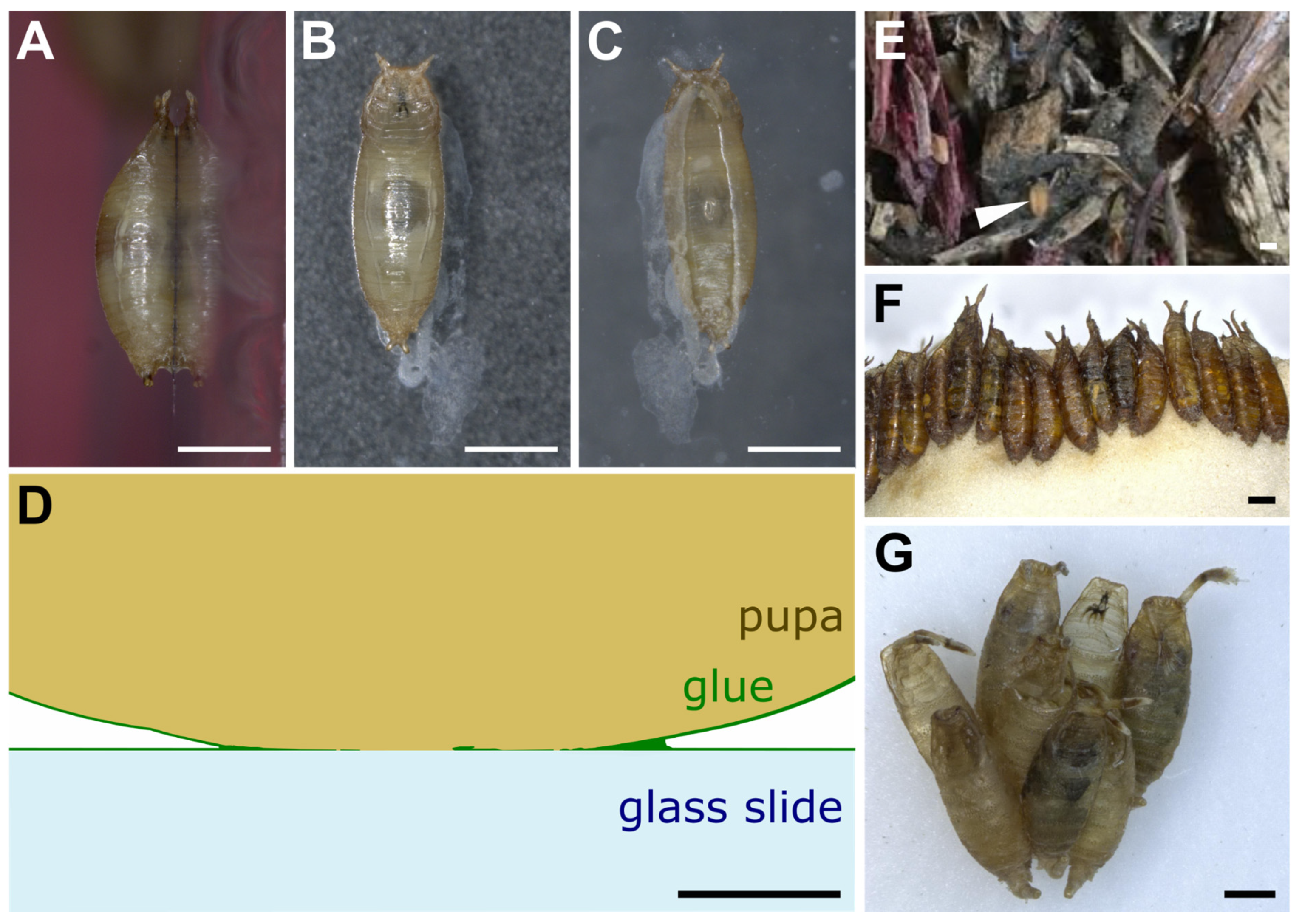
Figure 1. Drosophila pupae attached to various substrates. (A–C) D. melanogaster pupa attached with its own glue to a glass slide. Three pictures were taken of the same individual: (A) side view, (B) dorsal view, (C) ventral view throughout the glass slide. Anterior is up. White prints correspond to glue tracks on the glass slide or secretion from the larva before pupation. (D) Schematic transverse section of part of a D. melanogaster pupa attached to a glass slide with its own glue. Drawing based on confocal microscopy sections of Sgs3:GFP pupae obtained as in [21]. (E) First centimeters of soil made of wood chips from Bassevelle, France, where a Drosophila pupa (arrowhead) was found in July 2018. (F) Array of D. hydei pupae naturally attached to the plug within a laboratory vial. (G) Cluster of D. acanthoptera (Cornell University Drosophila Species Stock Center, stock #15090-1693.00) pupae found on the plastic wall of a laboratory vial. Scale bar is 1 mm in all panels except panel D, where it is 100 µm.
On the substrate near the posterior part of the animal is often found a whitish material that appears to be expelled by the intestine prior to metamorphosis and that mixes partly with the glue that is expectorated from the anterior part of the larva (Figure 1B,C) [21][22]. Whether this whitish material contributes to the adhesive properties of the glue is unknown.
In the wild and in the laboratory, pupae can be attached to the substrate either in isolation or in clusters (Figure 1D,E) [14][23][24]. When clustered, pupae are usually aligned with their anteroposterior axes pointing to the same direction (Figure 1D,E) [25]. In the wild, when pupating on Opuntia cactus, D. buzzatii and D. simulans tend to form species-specific aggregations in different locations on the cactus [15]. This clumping behavior, in which individuals of a given species closely group with each other, might improve the animals’ attachment, as their glue may combine with the glue of other already attached individuals.
Scanning electron microscopy reveals that the surface of the glue is uniformly smooth and that its internal aspect is complex and structured [21][22]. The glue seems to be organized in thin layers separated by air bubbles [21] and is made of a multidirectional arrangement of thick fibers of various densities ranging from 30 to 90 nm diameter [22]. The variability in inner glue fiber thickness may serve as an elastic buffer that can accommodate mechanical stress exerted onto the animal and may allow its firm attachment to the substrate [22].
3. Adhesive Properties of the Glue
The adhesion force at the bonding surface of a bioadhesive can be measured in the laboratory as the force required to detach one material from the other under the application of a shearing, tensile or peeling force [1]. Recently, three studies of one group and collaborators used an automated pull-off adhesion test program to evaluate the force required to detach pupae from a substrate [21][26][27]. Third instar wandering larvae were let to pupariate on glass slides and kept in a box with wet paper. Fifteen to twenty-one hours later, pull-off force assays were conducted on pupae naturally attached to glass slides with their own glue using a universal test machine with a 5 N force sensor covered with double-sided tape. The glass slide with a pupa attached on it was placed under the force sensor. The force sensor was moved down until reaching the pupa, pressing onto it until a determined maximal force of 0.07 N, stilled at 0.03 N for 10 s and finally moved up at a constant speed of 0.2 mm·s−1 until a determined position. During the assay, three variables were measured: time (seconds), position of the force sensor (extension in mm) and force (N). The maximal value of the force reached during the assay when the pupa detaches from the glass slide was considered as the glue adhesion force for the individual.
The pull-off force for D. melanogaster pupae on glass slides was found to range from 151 mN to 269 mN with an average of 217 mN (15,500 times the weight of a pupa) [21]. By dividing the force value by the area of the pupa–substrate interface, which is approximately 1.1 mm2, the adhesive strength is thus estimated at 137–244 kPa (1 Pa = 1 N/m2) [21]. Adhesive strengths of the same order of magnitude (hundreds of kilopascals) are found for commercial adhesive tapes and for the mussel-inspired epoxy bioadhesives (136 kPa) [28]. In comparison, the cyanoacrylates composing highly adhesive glues, known as Super Glue, have a lap-shear force 100 times higher, of about 13.7 MPa [29].
Interestingly, diverse substrates, non-coated, Poly-L-lysine-coated (PLL-coated), poly-L-lysine–polyethylene glycol-coated (PLL–PEG- coated) and oxygen-activated glass slides present very similar adhesion forces ranging from 184 mN to 229 mN. In these cases, the break most often occurs between the pupal case and the glue, indicating that the bond between the glue and the substrate is stronger than the bond between the glue and the animal. This also suggests that the assay measures the adhesion force between the animal and its bioadhesive and not with its pupariation substrate. This observation can explain the similarity in adhesion forces between different substrates. Similarly, with substrates of increasing roughness, the break usually occurs between the pupal case and the glue, and no significant amelioration of the adhesion is detected [21]. As a control, when a low-stickiness substrate such as polytetrafluoroethylene (PTFE, Teflon) is used as a pupariation substrate, during the assay the glue completely detaches from the substrate and remains on the pulled pupa, and the average adhesion force is significantly lower (42 mN).
Taken together, these results mean that the bond between the glue and many different types of substrates is stronger than 200 mN, except for Teflon. No effect of humidity, temperature, atmospheric pressure and age of the pupa was found on the adhesion force measure [21], but the ranges in temperature and age of the pupa were small (respectively, 23.5–27.9 °C and 3.5–23 h), so it is possible that more extreme temperatures and older pupae exhibit differences in adhesion forces.
Relatively high variation in adhesion forces was detected between pupae for the same substrate, even within the same strain, ranging for example from 80 mN to 430 mN between D. melanogaster individuals for glass slides [27]. This variation cannot be solely attributed to the measurement error, as the universal test machine has an accuracy of ±0.5%. The adhesion assay described here measures the adherence of naturally attached pupae, and it is possible that factors that are not controlled in the experiment greatly influence adhesion, such as animal size, shape, weight, position of the pupa on the substrate or the amount of glue produced. Ideally, it would be good to develop adhesion assays on extracted glue. Unfortunately, there is currently no means to trigger glue expectoration. A recent study [22] that managed to collect glue monitored the larvae under a stereomicroscope until glue expectoration and required action within a few seconds before the glue solidified completely, which is a time-consuming approach.
Comparison of 12 D. melanogaster lines from different geographical regions revealed that adhesion can also vary between strains of the same species [27]. Besides D. melanogaster, glue adhesion strength has been reported in only three Drosophila species so far [26]. D. simulans detach at a similar force (median of 234.2 mN) [26] to D. melanogaster (median of 217 mN) [21]. D. hydei have the highest force (median of 482.6 mN) and D. suzukii the lowest (median of 78.7 mN). Furthermore, the adhesion force correlates with the glue contact area between the pupa and the substrate for these three species [26] but not for D. melanogaster [21].
Noticeably, most Drosophila researchers who manipulated pupae in vials know from experience that the glue displays an interesting reversible adhesiveness property. Pupae can be detached from the glass or plastic vial to which they stick by adding a small drop of water, waiting about one minute for the glue to swell in the water and then using a small paintbrush to gently detach the pupa. Such detached pupae can then be placed in another location within the vial. When dried, the glue will strongly adhere again to the tube.
In conclusion, assays have been developed recently to evaluate the force of detachment of naturally glued pupae. These assays will be very useful in future years to assess the range of adhesion forces across various fly species, diverse substrates and various environmental conditions.
4. Production and Expectoration of the Glue
The glue is made of water and several proteins named glue proteins [30]. The production of glue proteins in D. melanogaster begins during the second half of the third larval instar with their synthesis in the endoplasmic reticulum, where they are folded and then transported to the Golgi apparatus via the formation of Tango1-mediated rings that act as docking points between the endoplasmic reticulum and Golgi [31]. There, some of the glue proteins are glycosylated, and all the glue proteins are packaged into vesicles, also named granules. As they leave the trans Golgi network, these granules are about 1 μm in diameter, and they will fuse with each other to give large mature granules, about 3 to 8 μm in diameter [32]. Each salivary gland in D. melanogaster contains between 2500 and 3000 individual secretory granules [19]. In the granules, three main ultrastructural components are observed: a paracrystalline component made of electron-dense filament bundles, electron-lucent discs and a fine particulate or electron-opaque matrix [19][33]. The formation, composition and properties of these individual components is starting to be studied [33]. Progressively, glue proteins appear to be densely packed and dehydrated in large vesicles, in a process involving granule acidification, chloride ions, calcium ions and glycosylation [33].
Four to five hours prior to expectoration, a pulse of ecdysone triggers exocytosis, and granules release their content into the salivary gland lumen in an actomyosin-dependent process [34][35][36]. Once the secretion begins, the paracrystalline structure is lost and the lumen is filled with an amorph secretion [37][38]. A rise in pH and disappearance of calcium ions lead to the unfolding and hydration of the granule contents with water coming from the hemolymph, increasing the total volume [39][40]. At the end of the third instar larval stage, the salivary gland becomes bloated and full of glue.
When a larva finds an appropriate substrate for pupariation, it expectorates the glue, and the content of the lumen of the salivary gland is expelled through the mouth [5]. The process of glue expectoration has been described recently in exquisite detail based on movies of D. melanogaster larvae expressing Sgs3:GFP fluorescent glue [41]. At the end of the larval stage, the larva everts its pair of anterior spiracles, which are respiratory openings through which air will pass during metamorphosis, and it moves less and less. The animal also acquires a characteristic barrel shape through increasingly strong whole-body contractions and then enters a tetanic contraction phase where ventral anterior segments contract and slightly arch the anterior half of the larva for 17–70 s. Then, an anterior peristaltic wave propagates from segment T2 to A2 in approximately 3 s, further squeezing the anterior segments. A few milliseconds later, the glue is expelled from the lumen of the salivary gland to the exterior of the animal. While the glue is being released, a series of coordinated peristaltic movements propagate forwards and backwards, starting from segment A2, and lead to the spreading of the glue throughout the whole body. Furthermore, during expectoration, the animal usually moves forward about half of its length, reaching its final pupariation site, where it typically waves its anterior end left and right a few times. From the tetanus phase to the head waving, about 60–70 s have elapsed. Then, occasional whole-body contractions occur for about 50 min: they help remodel the puparium shape and lead to the formation of the operculum (the part of the pupal case that will be opened up by the adult fly when it emerges at the end of metamorphosis), and the cuticle starts to harden. The same suite of behavioral events accompanying glue expectoration was observed in D. virilis [41], which diverged from D. melanogaster about 45 million years ago [42]. After expectoration, the glue is liquid and it hardens in a few seconds, depending on air humidity, and becomes completely dry and solid after 3–5 min [4][22]. The movements of the larva during expectoration allow the glue to completely wet the body and increase contact with the animal surface topography by flowing into the folds and crevices of the cuticle, thus maximizing adhesiveness between the animal and the substrate. The behavior of larvae that stick themselves to already attached pupae has not been described.
In summary, the stickiness of the pupae to their substrate results not only from the biochemical properties of the glue but also from the behavior of the larvae, including its body shape remodeling and its spreading of the glue via peristaltic movements. The glue proteins display remarkable properties, allowing them to be packed and dried into granules, fluidified in the gland lumen and then solidified in contact with air.
This entry is adapted from the peer-reviewed paper 10.3390/insects13080734
References
- Bianco-Peled, H.; Davidovich-Pinhas, M. Bioadhesion and Biomimetics: From Nature to Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-981-4463-99-7.
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255.
- Kord Forooshani, P.; Lee, B.P. Recent Approaches in Designing Bioadhesive Materials Inspired by Mussel Adhesive Protein. J. Polym. Sci. Part Polym. Chem. 2017, 55, 9–33.
- Fraenkel, G.; Brookes, V.J. The Process by which the Puparia of Many Species of Flies Become Fixed to a Substrate. Biol. Bull. 1953, 105, 442–449.
- Ashburner, M. Drosophila: A Laboratory Handbook, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2004.
- O’Grady, P.M.; DeSalle, R. Phylogeny of the Genus Drosophila. Genetics 2018, 209, 1–25.
- Markow, T.; O’Grady, P.M. Evolutionary Genetics of Reproductive Behavior in Drosophila: Connecting the Dots. Annu. Rev. Genet. 2005, 39, 263–291.
- Godoy-Herrera, R.; Cifuentes, L.; Díaz de Arcaya, M.F.; Fernández, M.; Fuentes, M.; Reyes, I.; Valderrama, C. The Behaviour of Drosophila Melanogaster Larvae during Pupation. Anim. Behav. 1989, 37, 820–829.
- Godoy-Herrera, R.; Silva-Cuadra, J.L. The Behavior of Sympatric Chilean Populations of Drosophila Larvae during Pupation. Genet. Mol. Biol. 1998, 21, 31–39.
- Vandal, N.B.; Siddalingamurthy, G.S.; Shivanna, N. Larval Pupation Site Preference on Fruit in Different Species of Drosophila. Entomol. Res. 2008, 38, 188–194.
- Carson, H.L. The Association between Drosophila Carcinophila Wheeler and Its Host, the Land Crab Gecarcinus ruricola (L.). Am. Midl. Nat. 1967, 78, 324–343.
- Carson, H.L.; Wheeler, M.R. Drosophila Endobranchia, a New Drosophilid1 Associated with Land Crabs in the West Indies. Ann. Entomol. Soc. Am. 1968, 61, 675–678.
- Sokolowski, M.B. Genetics and Ecology of Drosophila Melanogaster Larval Foraging and Pupation Behaviour. J. Insect Physiol. 1985, 31, 857–864.
- Beltramí, M.; Medina-Muñoz, M.C.; Arce, D.; Godoy-Herrera, R. Drosophila Pupation Behavior in the Wild. Evol. Ecol. 2010, 24, 347–358.
- Beltramí, M.; Medina-Muñoz, M.C.; Pino, F.D.; Ferveur, J.-F.; Godoy-Herrera, R. Chemical Cues Influence Pupation Behavior of Drosophila Simulans and Drosophila Buzzatii in Nature and in the Laboratory. PLoS ONE 2012, 7, e39393.
- Grossfield, J. Non-Sexual Behavior of Drosophila; Academic Press: London, UK, 1978; Volume 2, pp. 1–126.
- Woltz, J.M.; Lee, J.C. Pupation Behavior and Larval and Pupal Biocontrol of Drosophila Suzukii in the Field. Biol. Control 2017, 110, 62–69.
- Crosby, M.A.; Meyerowitz, E.M. Drosophila Glue Gene Sgs-3: Sequences Required for Puffing and Transcriptional Regulation. Dev. Biol. 1986, 118, 593–607.
- Farkaš, R. The Complex Secretions of the Salivary Glands of Drosophila Melanogaster, A Model System. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 557–600. ISBN 978-3-319-40738-8.
- Meyerowitz, E.M.; Crosby, M.A.; Garfinkel, M.D.; Martin, C.H.; Mathers, P.H.; Vijay Raghavan, K. The 68C Glue Puff of Drosophila. Cold Spring Harb. Symp. Quant. Biol. 1985, 50, 347–353.
- Borne, F.; Kovalev, A.; Gorb, S.; Courtier-Orgogozo, V. The Glue Produced by Drosophila melanogaster for Pupa Adhesion Is Universal. J. Exp. Biol. 2020, 223, jeb220608.
- Beňová-Liszeková, D.; Beňo, M.; Farkaš, R. A Protocol for Processing the Delicate Larval and Prepupal Salivary Glands of Drosophila for Scanning Electron Microscopy. Microsc. Res. Tech. 2019, 82, 1145–1156.
- Del Pino, F.; Jara, C.; Pino, L.; Godoy-Herrera, R. The Neuro-Ecology of Drosophila Pupation Behavior. PLoS ONE 2014, 9, e102159.
- Medina-Muñoz, M.C.; Godoy-Herrera, R. Dispersal and Prepupation Behavior of Chilean Sympatric Drosophila Species That Breed in the Same Site in Nature. Behav. Ecol. 2005, 16, 316–322.
- Ringo, J.; Dowse, H. Pupation Site Selection in Four Drosophilid Species: Aggregation and Contact. J. Insect Behav. 2012, 25, 578–589.
- Borne, F.; Prigent, S.R.; Molet, M.; Courtier-Orgogozo, V. Drosophila Glue Protects from Predation. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210088.
- Borne, F.; Kulathinal, R.J.; Courtier-Orgogozo, V. Glue Genes Are Subjected to Diverse Selective Forces during Drosophila Development. Genome Biol. Evol. 2021, 13, evab248.
- Du, D.; Chen, X.; Shi, C.; Zhang, Z.; Shi, D.; Kaneko, D.; Kaneko, T.; Hua, Z. Mussel-Inspired Epoxy Bioadhesive with Enhanced Interfacial Interactions for Wound Repair. Acta Biomater. 2021, 136, 223–232.
- Ebnesajjad, S.; Landrock, A.H. Characteristics of Adhesive Materials. In Adhesives Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2015; pp. 84–159. ISBN 978-0-323-35595-7.
- Kress, H. Temporal Relationships between Leaving Food, Ecdysone Release, Mucoprotein Extrusion and Puparium Formation in Drosophila Virilis. J. Insect Physiol. 1974, 20, 1041–1055.
- Reynolds, H.M.; Zhang, L.; Tran, D.T.; Ten Hagen, K.G. Tango1 Coordinates the Formation of Endoplasmic Reticulum/Golgi Docking Sites to Mediate Secretory Granule Formation. J. Biol. Chem. 2019, 294, 19498–19510.
- Ji, S.; Samara, N.L.; Revoredo, L.; Zhang, L.; Tran, D.T.; Muirhead, K.; Tabak, L.A.; Ten Hagen, K.G. A Molecular Switch Orchestrates Enzyme Specificity and Secretory Granule Morphology. Nat. Commun. 2018, 9, 3508.
- Syed, Z.A.; Zhang, L.; Tran, D.T.; Bleck, C.K.E.; Hagen, K.G.T. Orchestrated Restructuring Events During Secretory Granule Maturation Mediate Intragranular Cargo Segregation. bioRxiv 2022. bioRxiv:2021.08.16.456250.
- Rousso, T.; Schejter, E.D.; Shilo, B.-Z. Orchestrated Content Release from Drosophila Glue-Protein Vesicles by a Contractile Actomyosin Network. Nat. Cell Biol. 2016, 18, 181–190.
- Duan, J.; Zhao, Y.; Li, H.; Habernig, L.; Gordon, M.D.; Miao, X.; Engström, Y.; Büttner, S. Bab2 Functions as an Ecdysone-Responsive Transcriptional Repressor during Drosophila Development. Cell Rep. 2020, 32, 107972.
- Tran, D.T.; Masedunskas, A.; Weigert, R.; Ten Hagen, K.G. Arp2/3-Mediated F-Actin Formation Controls Regulated Exocytosis in Vivo. Nat. Commun. 2015, 6, 10098.
- von Gaudecker, B. Der Strukturwandel der larvalen Speicheldrüse vonDrosophila melanogaster. Z. Für Zellforsch. Mikrosk. Anat. 1972, 127, 50–86.
- Farkaš, R.; Šuťáková, G. Ultrastructural Changes of Drosophila Larval and Prepupal Salivary Glands Cultured in Vitro with Ecdysone. Vitro Cell. Dev. Biol.—Anim. 1998, 34, 813–823.
- Syed, Z.A.; Zhang, L.; Ten Hagen, K.G. In Vivo Models of Mucin Biosynthesis and Function. Adv. Drug Deliv. Rev. 2022, 184, 114182.
- Lane, N.J.; Carter, Y.R.; Ashburner, M. Puffs and Salivary Gland Function: The Fine Structure of the Larval and Prepupal Salivary Glands OfDrosophila Melanogaster. Wilhelm Roux Arch. Für Entwickl. Org. 1972, 169, 216–238.
- Heredia, F.; Volonté, Y.; Pereirinha, J.; Fernandez-Acosta, M.; Casimiro, A.P.; Belém, C.G.; Viegas, F.; Tanaka, K.; Menezes, J.; Arana, M.; et al. The Steroid-Hormone Ecdysone Coordinates Parallel Pupariation Neuromotor and Morphogenetic Subprograms Via Epider-Mis-To-Neuron Dilp8-Lgr3 Signal Induction. Nat. Commun. 2021, 12, 1–20.
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819.
This entry is offline, you can click here to edit this entry!
