Metal nanoparticles (NPs) have received much attention for potential applications in medicine (mainly in oncology, radiology and infectiology), due to their intriguing chemical, electronical, catalytical, and optical properties such as surface plasmon resonance (SPR) effect. They also offer ease in controlled synthesis and surface modification (e.g., tailored properties conferred by capping/protecting agents including N-, P-, COOH-, SH-containing molecules and polymers such as thiol, disulfide, ammonium, amine, and multidentate carboxylate), which allows (i) tuning their size and shape (e.g., star-shaped and/or branched) (ii) improving their stability, monodispersity, chemical miscibility, and activity, (iii) avoiding their aggregation and oxidation over time, (iv) increasing their yield and purity. The bottom-up approach, where the metal ions are reduced in the NPs grown in the presence of capping ligands, has been widely used compared to the top-down approach. Besides the physical and chemical synthesis methods, the biological method is gaining much consideration. Indeed, several drawbacks have been reported for the synthesis of NPs via physical (e.g., irradiation, ultrasonication) and chemical (e.g., electrochemisty, reduction by chemicals such as trisodium citrate or ascorbic acid) methods (e.g., cost, and/ortoxicity due to use of hazardous solvents, low production rate, use of huge amount of energy). However, (organic or inorganic) eco-friendly NPs synthesis exhibits a sustainable, safe, and economical solution. Thereby, a relatively new trend for fast and valuable NPs synthesis from (live or dead) algae (i.e., microalgae, macroalgae and cyanobacteria) has been observed, especially because of its massive presence on the Earth's crust and their unique properties (e.g., capacity to accumulate and reduce metallic ions, fast propagation). This work highlights affordable, fast, eco-friendly, efficient and safe strategies to produce nanoparticles for theranostic purposes.
- Nanomedicine
- Green nanobiotechnology
- algae
- Nanoparticles
- Drug delivery
- Nanotheranostics
- Oncology
- Microbiology
1. Introduction
Nanotechnology is a vibrant, revolutionary, and fast-developing multidisciplinary field that deals at nanoscopic scale (10−9 m), impacting the environment, health, and socio-economy for more than two decades [1][2][3][4]. The resulting (natural, engineered or incidental) nanomaterials (e.g., nanoparticles (NPs)) usually display a dimension below 100 nm along with unique physical–chemical–biological properties (e.g., large surface-to-volume ratio, surface functionalization, controlled targeting and release) compared to their counterpart bulk materials. These interesting features make them incredibly attractive tools for applications in various fields (e.g., cosmetology, pharmacy, biotechnology, chemistry, or agriculture).
Actually, metal and non-metal NPs synthesized from plants (e.g., seaweeds) and microorganisms (e.g., bacteria) have attained more attention than classical physical and chemical synthesis routes mainly because of their (i) green assembly during the biosynthesis that uses clean energy processes regulated naturally, which subsequently overcomes the health and environmental toxicity; (ii) various attributes that contrast with those of them in the massive structure; (iii) peerless characteristics that drastically depend on the type (e.g., morphology/shape, size distribution, composition, and stability) and the dose, which can both impact on toxicity; (iv) cost-effectiveness; (v) eligibility for usage in a wide range of activities encompassing cosmetics, theranostics, food and textile fields [1][2][3][4].
Importantly, a recent trend of biologically synthesizing NPs using algal species (e.g., Chlorophyceae (green), Phaeophyceae (brown), Cyanophyceae (blue green), Rhodophyceae (red), Diatoms) is developing [5]. This tremendous and increasing interest is mainly due to the (i) ease of handling algae; (ii) capacity of algae to absorb/accumulate inorganic metallic ions; (iii) alternative strategy for cost-effective synthesis; and (iv) naturally eco-friendly, fast and healthier synthesis methods [5].
Algae represent an autotrophic (i.e., producers of their own food using light, water, carbon dioxide, or other chemicals) and polyphyletic (i.e., large and diverse) group of photosynthetic eukaryotic organisms. They are classified as microalgae (unicellular such as diatoms or multicellular) and macroalgae (seaweeds), primarily based on their morphological features, and can be found in marine and freshwater environments or over moist rocks [6]. These aquatic plants are simple and non-flowering. Indeed, although algae contain chlorophyll, they are lacking various structures (e.g., true stems, roots, leaves, and vascular tissue) that characterize the land plants (e.g., bryophytes, tracheophytes) [7]. Even so, they play a key role in the aquatic ecosystems (outside of their possible toxic blooming, which, interestingly, can be controlled by algal-mediated NPs) and represent an economically valuable biomass source for various applications (e.g., agricultural, aquacultural, pharmaceutical, cosmetical, biotechnological, energetical, and nanotechnological) [8][9].
2. Types of Nanoparticles and Applications
There are two kinds of NPs that can be synthesized using algae: the organic and inorganic ones.
Organic NPs include poly-ɛ-lysine (ɛ-PL), chitosan (CS), cationic quaternary polyelectrolytes and quaternary ammonium compounds. Generally, these organic NPs are weakly stable at high temperatures compared to that of inorganic NPs, favoring inorganic NPs as antimicrobial polymers [10]. Even so, ε-PL is a L-lysine cationic homopeptide which is very efficient against Gram-positive bacteria and spores of B. coagulans, B. subtilis, and B. stearothermophilus [11][12]. CSNPs possess a wide spectrum of antimicrobial activity against viruses, bacteria, and fungi. They are nontoxic, biocompatible and can serve as absorption enhancers [13][14]. Cationic quaternary polyelectrolytes represent by-products of acrylic and methacrylic compounds. Due to their structural versatility, these molecules have a broad variety of biological applications (e.g., as antimicrobials) when factors such as their hydrophobicity, surface charge, molecular weight are altered [15]. Quaternary ammonium compounds are eminent disinfectants and rely on chain length for their antimicrobial properties [16]. Interestingly, the synthesis of organic NPs by algae has been poorly studied so far; This may be due to the lack of stability of the organic NPs at high temperature. Recently, Tiburu et al. (2017) investigated the formation of CSNPs by isolating deacetylated chitin from freshwater green algae [17]. The chitin was then converted into CS by mixing it with caustic soda and heating it by indirect steam. The resulting CSNPs were of an orthorhombic structure as revealed by x-ray powder diffraction (XRD) analysis.
Inorganic NPs, which include Ag, zinc oxide (ZnO), copper oxide (CuO), Au, and iron oxide (magnetite Fe3O4 and/or its oxidized form maghemite γ-Fe2O3), are the focus of this article. Metal NPs are the most studied nanomaterials because they display unique catalytic, electronic, and optical properties [18]. They also offer ease in controlled synthesis and surface modification, which allows tuning their size and shape-dependent properties [18]. It is now established that AgNPs possess strong antimicrobial properties against many fungi, viruses and bacteria because of their action as a photocatalyst and the capability to produce reactive oxygen species (ROS) [19][20][21][22]. ZnONPs are low cost antibacterial agents that can restrict the growth of a wide array of pathogenic bacteria [23]. They show anti-biofilm activity and can also block UV-rays, thus representing an effective coating material in medical and food materials [24]. CuONPs are used as antibacterial agents but they are less efficient than AgNPs or ZnONPs [25]. Their antibacterial activity is exerted through their capacity to disrupt cell membranes and produce ROS [26]. AuNPs are considered as non-toxic and are mainly used for gene delivery applications, biosensing and cancer therapy [27][28][29]. Iron oxide NPs are much applied in magnetic resonance imaging (MRI), immunoassays, tissue repair, and as effective agents in chemotherapy [30][31].
3. General Methods for Synthesizing Nanoparticles with Tailored Properties
NPs synthesis can follow two different pathways by using either the technique "bottom-up" or by applying the technique "top-down". The "bottom-up" technique is also called the self-assembly method because the initial synthesized NPs combine to form a structure or cluster by using chemical or biological methods. In "top-down" technique (e.g., ion etching, lithography), an appropriate bulk content is reduced to fragments using either chemical or physical processes.
NPs obtained from the "bottom-up" method (e.g., chemical/physical vapor deposition, electrochemistry) are preferred since they display more homogenous chemical composition and lesser defects than those resulting from "top-down" methods that elicit imperfect surface structures. These defected surface structures can have harmful impacts on the physical and chemical properties of NPs [32].
Wet-chemical processes are commonly used methods for synthesizing NPs, and implicate that NPs are grown in liquid media in the presence of several reactants, reducing and stabilizing/protecting agents (e.g., capping agents/ligands). Typically, NPs are synthesized by chemical reduction either by organic or inorganic reducing agents, via the colloidal route or by sol-gel method [32][33][34]. Although these methods yield high production of NPs at a low cost, they also contain some drawbacks (e.g., use of hazardous solvents, processing of harmful by-products and contamination from precursor chemicals) [32].
Physical synthesis of NPs involves various methods such as ultrasonication, electron beam, ion implantation, laser radiation, spray pyrolysis and vapor phase [34]. However, these physical methods have some drawbacks that make them less suitable for synthesizing NPs (e.g., high cost, low production rate, use of a huge amount of energy for maintaining the high temperature and pressure) [32].
To eliminate the drawbacks of physical and chemical methods used in the synthesis of NPs, an increasing interest is observed for green NPs synthesis using biological entities (e.g., microorganisms including bacteria, fungi, and yeast, microalgae or plant extracts including from macroalgae), which are considered as safe for health while minimizing the scarcity of energy resources [5][32].
Eventually, the choice of preparation procedure not only shall depend on the physical and chemical characteristics required on the final product (e.g., size (usually < 100 nm), dispersion, shape (e.g., spherical, star-shaped, or branched NPs), chemical miscibility, optical properties (surface plasmon resonance (SPR) effect), but also shall consider environmental aspects.
4. Key Factors Governing Nanoparticles Synthesis
Several chemical, physical, and biological controlling factors are responsible for the proper, efficient, and optimal NPs (bio)synthesis process. These include pH, temperature, metal ion concentrations, reactant concentrations, reaction time, stirring rate, incubation time, capping agents, and the type of microorganism or plant extract used. Most of these factors, if not all, can have an impact on the stability, size, and shape of the NPs. Besides, it is well known that the toxicity of nanomaterials essentially depends on the structural features such as the size, shape, composition, and surface chemistry.
5. Algal-Mediated Inorganic Nanoparticles Synthesis Methods
As previously evoked, algae are rich in polymeric molecules (e.g., polysaccharides) and can hyperaccumulate heavy metal ions and remodel them into malleable forms by (bio) reduction process. Algal extracts typically consist of pigments (e.g., chlorophylls, carotenoids, phycobilins), carbohydrates, proteins, minerals, polyunsaturated fatty acids (PUFAs), and other bioactive compounds such as antioxidants (e.g., polyphenols and tocopherols) that may be used as stabilizing/capping and reducing agents [35]. Moreover, the phycosynthesis of NPs takes less time compared to that of their biosynthetic entities [36]. Due to these overall properties, live or dead algae are used as a model organism for the eco-friendly synthesis procedure of bionanomaterials, such as metallic NPs [37]. In this routine, the NPs formation is followed by UV-Vis absorption spectroscopy and TEM, while the functional groups involved in the bioreduction are studied by FTIR [37].
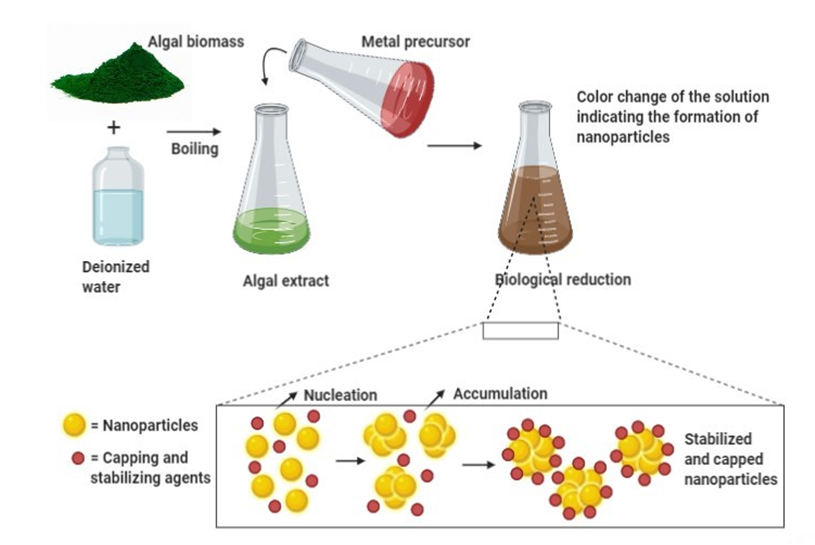
Algal-mediated synthesis of inorganic NPs, such as Ag and Au, which are the most investigated noble metals to be used by the algal biomass for producing NPs, can be obtained by the three subsequent steps (Figure 1) [36]: (i) heating or boiling algal extract after mixing it with water or an organic solvent for a certain time period; (ii) preparation of molar ionic metallic solutions; (iii) exposure of the algal extract to the molar solution of the (noble) metal ion precursor in a flask under (or not) continuous stirring for a certain time period. It is worth noting that the final reaction results in a color change that determines the nucleation in which the adjacent nucleon particles bind together to form thermodynamically stable NPs of varying shapes and sizes [38]. For instance, Rajeshkumar et al. (2012) synthesized AgNPs by using aquatic brown alga, Turbinaria conoides (J.Agardh) [39]. The alga was first cleaned and made into fine powder. A specific amount of algal extract was then added into a metal precursor of AgNO3 solution and stored at ambient temperature under mechanical stirring. The change of color from brown to dark brown showed the formation of NPs, which displayed spherical morphology with a mean particle size of 96 nm.
Algal-mediated synthesis of inorganic NPs can either be achieved intracellularly or extracellularly [40]. In intracellular NPs synthesis, algal biomass is first collected and washed with distilled water. The biomass (live algae) is then treated with specific metallic solutions such as AgNO3 solution. The mixture is subsequently incubated at specific temperature, pH, and time conditions for bioreduction. It is eventually sonicated and centrifuged to yield the harvested stable NPs. In extracellular synthesis of NPs, algal biomass is first collected and washed with distilled water. Then, the following three methods are commonly used for the further procedure: (i) the algal biomass (dead algae) is dried under shadow for a specific time and the dried powder is treated with distilled water and filtered out; (ii) the algal biomass is sonicated with distilled water to obtain a cell-free extract; or (iii) the algal biomass is washed with distilled water and incubated for a few hours (8–16 h) and the obtained product is then filtered.
Besides, in addition to the phycosynthesis of metallic NPs via an enzyme (intracellular nitrogenase)-mediated route for the metal (e.g., Ag, Au) reduction in cyanobacteria (e.g., Calothrix pulvinata) [41], recent studies report an innovative method, namely ultrasound irradiation-assisted synthesis (UIAS) of AgNPs and AuNPs, using the cyanobacteria Calothrix (C.Agardh ex Bornet & Flahault) [42][43]. This eco-friendly and economical technique permits the acceleration of a wide range of chemical reactions and extraction procedures thanks to resulting cavitational collapses. Indeed, cavitational collapse produces intense local heating (~5000 °C) and high pressures (~2000 atmospheres) in the liquid reaction mixture, with noticeably short lifetimes [42][43].
Figure 1. General mechanism for algal-mediated synthesis of inorganic/metallic nanoparticles.
In this illustration, algal biomass is first combined with deionized water and boiled at a certain temperature. The obtained algal extract is then treated with a metal precursor and incubated for a certain time at ambient temperature. The change of color indicates the formation of NPs. During the bioreduction of metal ions, the subsequent processes of nucleation and condensation (accumulation) assures the formation (growth) of stabilized NPs surrounded by capping agents. Then, several physical techniques can be used to characterize biosynthesized NPs. Thereby, the solution color change and SPR can be observed by UV-Vis spectroscopy, while the size, shape, and morphology of NPs can be determined by Dynamic Light Scattering (DLS) and/or Scanning Electron Microscopy (SEM) and/or TEM. In addition, their structure (functional groups) can be unraveled by FTIR spectroscopy, and their crystalline nature can be assessed by XRD spectroscopy.
6. Major Bioapplications and Underlying Molecular Mechanisms of Algal-Mediated Synthesis of Metallic NPs
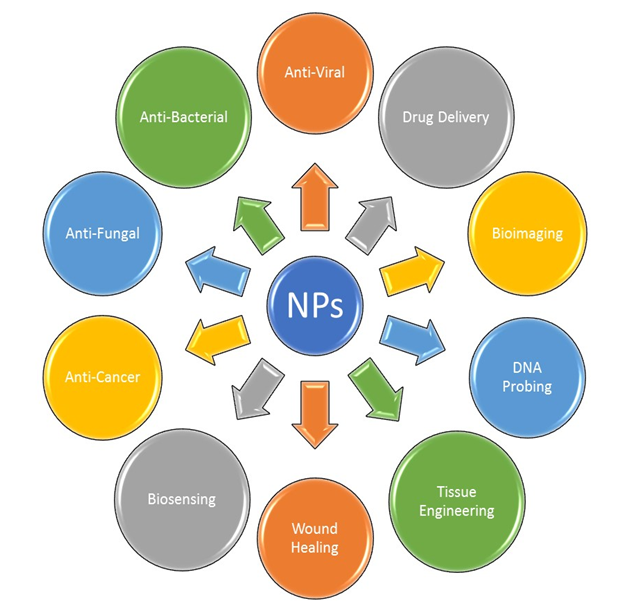
There are several applications reported for the algal-mediated synthesis of inorganic NPs. Metallic NPs and NPs, such as AgNPs and AuNPs, have been broadly considered for use in applications in a different scope of biotechnological, medical, cosmetical and pharmaceutical fields. The main applications of these NPs in the fields of medicine and biotechnology are illustrated in Figure 2.
Figure 2. Major applications of metallic NPs.
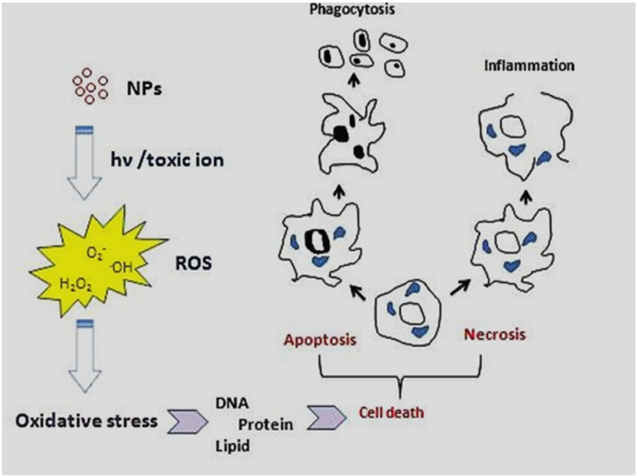
The physicochemical reactivity of (metallic) NPs leads to oxidative stress due to the resulting formation of free radicals or ROS (e.g., superoxide radical anions and hydroxyl radicals) directly or indirectly (i.e., through activation of oxidative enzymatic pathways), as shown in Figure 3. Some metal oxide NPs, such as titanium dioxide NPs (TiO2NPs) used in cosmetics, food additives and cancer therapy, are water-insoluble but would induce enhanced toxicity effects (e.g., oxidative stress, phototoxicity, genotoxicity and immunotoxicity), especially at concentrations higher than 100 μg/mL [44]. Despite other NPs such as ZnO, TiO2NPs do not release toxic ions hence toxicity of these NPs (i.e., ROS, mitochondrial depolarization, plasma membrane leakage, intracellular calcium influx and cytokine release) could be attributed to their size-dependent interaction/adsorption with intracellular biomolecules [44]. Additionally, it was shown that phototoxicity of these NPs could be decreased via surface coating with CS because of the prevention of biomolecule adsorption and hydroxyl radicals (.OH) production in the photoactivation process [44].
Figure 3. Major molecular and cellular mechanisms involved in the toxicity of (metallic) NPs [44]. (Reproduced with permission).
7. Conclusions and Perspectives
Here, we highlighted the biological method of synthesis of metallic NPs using algae. A special emphasis was given to AgNPs and AuNPs because of their unique features that have attracted much scientific and technologic interests. We described the main factors influencing a stable and efficient biosynthesis, as well as the most used algae for such purposes. We aimed to demonstrate the production of different sized NPs with many desirable shapes using various species of algae. We also provide some applications of metallic NPs in the field of nanomedicine, and their underlying main molecular mechanism-induced cytotoxicity. It is worth noting that algal-mediated NPs synthesis is a fast, cost-effective, and efficient strategy that has opened a way for nanotechnologists to produce desirable nanomaterials with clean energy processes. Nevertheless, there is a need to explore more in this field before these eco-friendly NPs are safely translated in medicine.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering7040129
References
- Batool, A.; Menaa, F.; Ali, K.B.; Uzair, B.; Menaa, B. Progress and prospects in translating nanobiotechnology in medical theranostics. Curr. Nanosci. 2019, 15, 1–23.
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774.
- Menaa, F. When pharma meets nano or the emerging era of nanopharmaceuticals. Pharm. Anal. Acta 2013, 4, 223, doi:10.4172/2153-2435.1000223.
- Menaa, B. The importance of nanotechnology in biomedical sciences. J. Biotechnol. Biomater. 2011, 1, 105e, doi:10.4172/2155-952X.1000105e.
- Tripathi, D.K.; Ahmad, P.; Sharma, S.; Chauhan, D.K.; Dubey, N.K. Nanomaterials in Plants, Algae, and Microorganisms: Concepts and Controversies: Volume 1; Academic Press: Cambridge, MA, USA, 2017.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Borghans, L.; Duckworth, A.L.; Heckman, J.J.; Ter Weel, B. The economics and psychology of personality traits. J. Hum. Resour. 2008, 43, 972–1059.
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415.
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36, doi:10.1186/s12934-018-0879-x.
- LewisOscar, F.; Vismaya, S.; Arunkumar, M.; Thajuddin, N.; Dhanasekaran, D.; Nithya, C. Algal nanoparticles: Synthesis and biotechnological potentials. Chapter. Algae Org. Imminent Biotechnol. 2016, 7, 157–182, doi:10.5772/62909.
- Hiraki, J. Basic and applied studies on ε-polylysine. J. Antibact Antifung. Agents 1995, 23, 349–354.
- Patel, J.K.; Sutariya, V.; Kanwar, J.R.; Pathak, Y.V. Drug delivery for the Retina and Posterior Segment Disease, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; p. 494, IBSN 978-3-319-95807-1.
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly (vinyl alcohol) nanofibers against staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2020, 144, 921–931.
- Chung, Y.-C.; Wang, H.-L.; Chen, Y.-M.; Li, S.-L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184.
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339.
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.Based Complementary Altern. Med. 2015, 2015, 246012, doi:10.1155/2015/246012.
- Tiburu, E.K.; Salifu, A.; Aidoo, E.O.; Fleischer, H.N.; Manu, G.; Yaya, A.; Zhou, H.; Efavi, J.K. Formation of chitosan nanoparticles using deacetylated chitin isolated from freshwater algae and locally synthesized zeolite A and their influence on cancer cell growth. Proc. J. Nano Res. 2017, 48, 156–170.
- San, K.A.; Shon, Y.-S. Synthesis of alkanethiolate-capped metal nanoparticles using alkyl thiosulfate ligand precursors: A method to generate promising reagents for selective catalysis. Nanomaterials 2018, 8, 346, doi:10.3390/nano8050346.
- Garipov, I.T.; Khaydarov, R.R.; Gapurova, O.U.; Efimova, I.L.; Evgrafova, S.Y. Silver nanoparticles as a new generation of antimicrobial prophylaxis. J. Sib. Fed. Univ. Biol. 2019, 12, 266–276.
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83.
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Photocatalytic action of AgCl nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B Biol. 2014, 138, 302–306.
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J. Growth kinetics and mechanistic action of reactive oxygen species released by silver nanoparticles from aspergillus niger on Escherichia coli. Biomed Res. Int. 2014, 2014, doi:10.1155/2014/753419.
- Jin, T.; Sun, D.; Su, J.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, salmonella enteritidis, and Escherichia coli O157: H7. J. Food Sci. 2009, 74, M46–M52.
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401.
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716.
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815.
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315.
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491.
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomed. (Lond) 2007, 2, 681–693.
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021.
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46.
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262.
- Menaa, B.; Miyagawa, Y.; Takahashi, M.; Herrero, M.; Rives, V.; Menaa, F.; Eggers, D.K. Bioencapsulation of apomyoglobin in nanoporous organosilica sol-gel glasses: Influence of the siloxane network on the conformation and stability of a model protein. Biopolym. Orig. Res. Biomol. 2009, 91, 895–906.
- Sharma, G.; Pandey, S.; Ghatak, S.; Watal, G.; Rai, P.K. Potential of spectroscopic techniques in the characterization of “green nanomaterials”. In Nanomaterials in Plants, Algae, and Microorganisms; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–77.
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656, doi:10.1016/j.mimet.2019.105656.
- Vincy, W.; Mahathalana, T.J.; Sukumaran, S.; Jeeva, S. Algae as a source for synthesis of nanoparticles-a review. Int. J. Latest Trends Eng. Technol. 2017, Chapter 7, 5–9. http://dx.doi.org/10.5772/62909
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnology 2013, 7, 109–116.
- Rai, M.; Posten, C. Green Biosynthesis of Nanoparticles: Mechanisms and Applications; CABI: Wallingford, CT, USA, 2013; p. 248.
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int. J. Pharma Bio Sci. 2012, 3, 502–510.
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology-a review. Green Chem. 2017, 19, 552–587.
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djedjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708.
- Kumar, B.; Smita, K.; Sánchez, E.; Guerra, S.; Cumbal, L. Nanotechnology ecofriendly ultrasound-assisted rapid synthesis of gold nanoparticles using calothrix algae. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025013.
- Kumar, B.; Cumbal, L.; Debut, A. Phycosynthesis of silver nanoparticles using calothrix algae through ultrasonic method. In Proceedings of the XI Congreso de Ciencia Y Tecnologia ESPE, Sangolqui, Ecuador, 2016; pp. 213–216.
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv. Pharm. Bull. 2015, 5, 447, doi:10.15171/apb.2015.061.