Atypical atrial flutters (AAFL) are difficult-to-manage atrial arrhythmias, yet potentially amenable to effective radiofrequency catheter ablation (CA). However, data on CA feasibility are only sparingly reported in the literature in different clinical settings, such as AAFL related to surgical correction of congenital heart disease.
- atypical atrial flutter
- atrial fibrillation
- catheter ablation
1. Introduction
2. Clinical Settings Associated with Atypical Atrial Flutters
2.1. Surgical Correction for Congenital Heart Disease
2.2. Cardiac Surgery for Acquired Heart Disease
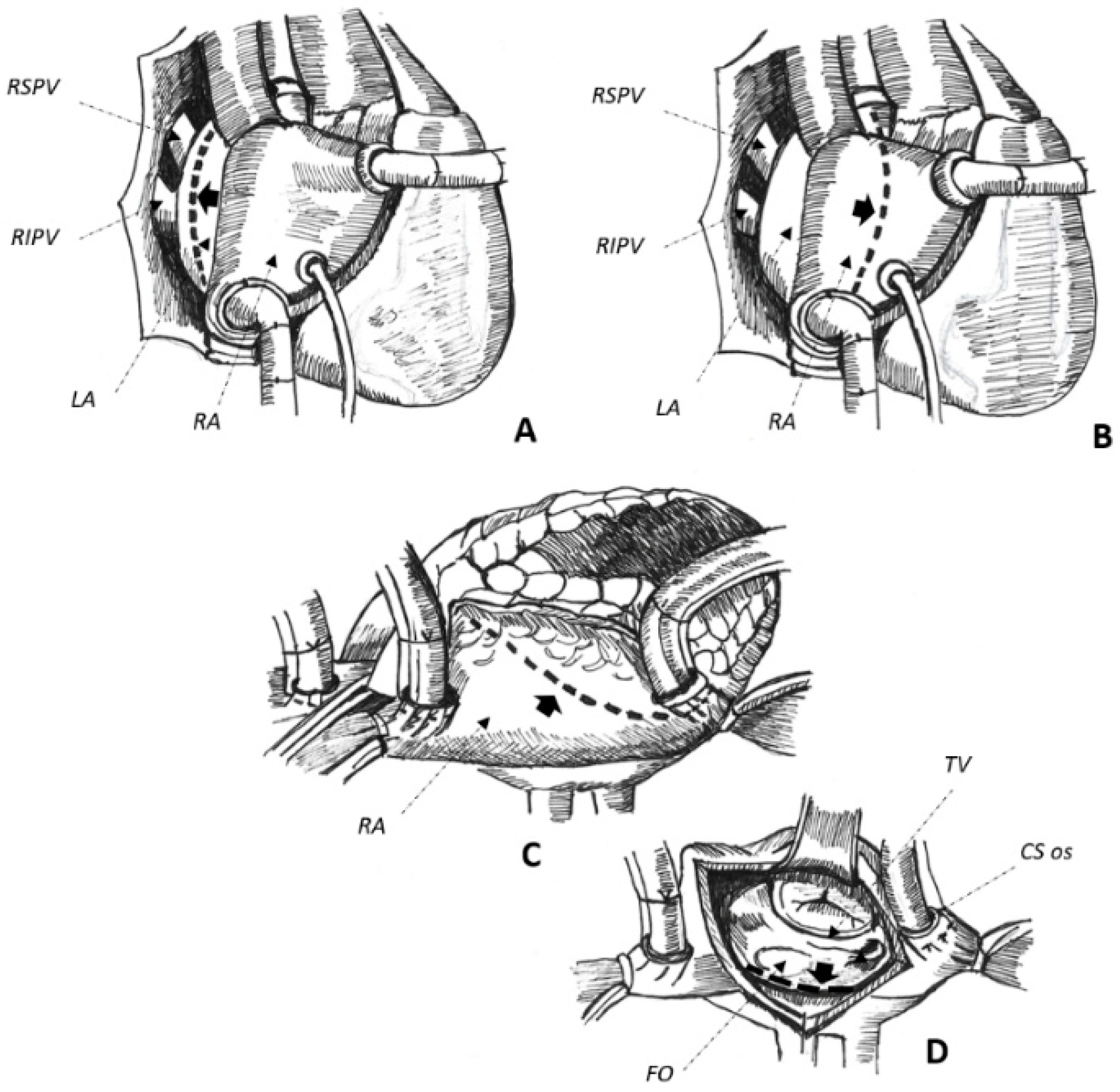
2.3. Non-Surgical Pulmonary Vein Isolation
2.4. Absence of Manifest Structural Heart Disease
3. Overall Peri-Procedure Feasibility
4. Maintenance of Sinus Rhythm after a Successful Procedure
AAFL recurrence is observed in up to 62% of cases after a single CA procedure with an overall SR maintenance as low as 38% on/off AAD after a variable follow-up duration, spanning from 7 ± 3 [43] to 37 ± 15 [5] months. Data on whether patients were on AAD before the procedure and at follow-up was not available in most of the studies, and the effect of AAD is therefore unclear in this setting.
The older the publication date, the greater the incidence of arrhythmia recurrence. This would suggest that the recent implementation of dedicated mapping tools [39] and irrigated-tip catheters [37][49] could help the cardiac electrophysiologist to achieve a greater long-term SR maintenance after an initially successful CA procedure [39][45]. The adoption of dedicated, tachycardia-oriented strategies for mapping and ablation of AAFL seem associated with even better results [35][45]. However, the greater the complexity of the atrial substrate to ablate, the higher the incidence of arrhythmia recurrence at follow-up. The worst long-term clinical outcome is commonly seen in patients with surgically corrected CHD (46–52% AAFL recurrence) [31][32], with better results observed after PVI (16–28%) [19][36] or in patients with apparently normal hearts (9–25% of tachycardia recurrence) [5][28].
5. The Winding Path to Improve the Procedure and the Overall Clinical Outcome
This entry is adapted from the peer-reviewed paper 10.3390/jcm11123323
References
- Bun, S.-S.; Latcu, D.G.; Marchlinski, F.; Saoudi, N. Atrial flutter: More than just one of a kind. Eur. Heart J. 2015, 36, 2356–2363.
- Twomey, D.; Sanders, P.; Roberts-Thomson, K.C. Atrial Macroreentry in Congenital Heart Disease. Curr. Cardiol. Rev. 2015, 11, 141–148.
- Enriquez, A.; Santangeli, P.; Zado, E.S.; Liang, J.; Castro, S.; Garcia, F.C.; Schaller, R.D.; Supple, G.E.; Frankel, D.S.; Callans, D.J.; et al. Postoperative atrial tachycardias after mitral valve surgery: Mechanisms and outcomes of catheter ablation. Heart Rhythm 2017, 14, 520–526.
- Deisenhofer, I.; Estner, H.; Zrenner, B.; Schreieck, J.; Weyerbrock, S.; Hessling, G.; Scharf, K.; Karch, M.R.; Schmitt, C. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: Incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 2006, 8, 573–582.
- Fiala, M.; Chovančík, J.; Neuwirth, R.; Nevřalová, R.; Jiravský, O.; Škňouřil, L.; Dorda, M.; Januška, J.; Vodzinská, A.; Černý, J.; et al. Atrial macroreentry tachycardia in patients without obvious structural heart disease or previous cardiac surgical or catheter intervention: Characterization of arrhythmogenic substrates, reentry circuits, and results of catheter ablation. J. Cardiovasc. Electrophysiol. 2007, 18, 824–832.
- Natale, A.; Newby, K.H.; Pisanó, E.; Leonelli, F.; Fanelli, R.; Potenza, D.; Beheiry, S.; Tomassoni, G. Prospective randomized comparison of antiarrhythmic therapy versus first- line radiofrequency ablation in patients with atrial flutter. J. Am. Coll. Cardiol. 2000, 35, 1898–1904.
- Derval, N.; Takigawa, M.; Frontera, A.; Mahida, S.; Konstantinos, V.; Denis, A.; Duchateau, J.; Pillois, X.; Yamashita, S.; Berte, B.; et al. Characterization of Complex Atrial Tachycardia in Patients with Previous Atrial Interventions Using High-Resolution Mapping. JACC Clin. Electrophysiol. 2020, 6, 815–826.
- Pap, R.; Kohári, M.; Makai, A.; Bencsik, G.; Traykov, V.B.; Gallardo, R.; Klausz, G.; Zsuzsanna, K.; Forster, T.; Sághy, L. Surgical technique and the mechanism of atrial tachycardia late after open heart surgery. J. Interv. Card. Electrophysiol. 2012, 35, 127–135.
- Scaglione, M.; Caponi, D.; Ebrille, E.; Di Donna, P.; Di Clemente, F.; Battaglia, A.; Raimondo, C.; Appendino, M.; Gaita, F. Very long-term results of electroanatomic-guided radiofrequency ablation of atrial arrhythmias in patients with surgically corrected atrial septal defect. Europace 2014, 16, 1800–1807.
- Kalman, J.M.; VanHare, G.F.; Olgin, J.E.; Saxon, L.A.; Stark, S.I.; Lesh, M.D. Ablation of ‘Incisional’ Reentrant Atrial Tachycardia Complicating Surgery for Congenital Heart Disease. Circulation 1996, 93, 502–512.
- Abrams, D.; Schilling, R. Mechanism and mapping of atrial arrhythmia in the modified Fontan circulation. Heart Rhythm 2005, 2, 1138–1144.
- Li, D.; Fan, Q.; Hirata, Y.; Ono, M.; An, Q. Arrhythmias After Fontan Operation with Intra-atrial Lateral Tunnel Versus Extra-cardiac Conduit: A Systematic Review and Meta-analysis. Pediatr. Cardiol. 2017, 8, 873–880.
- Markowitz, S.M.; Brodman, R.F.; Stein, K.M.; Mittal, S.; Slotwiner, D.J.; Iwai, S.; Das, M.K.; Lerman, B.B. Lesional tachycardias related to mitral valve surgery. J. Am. Coll. Cardiol. 2002, 39, 1973–1983.
- Marazzato, J.; Cappabianca, G.; Angeli, F.; Crippa, M.; Golino, M.; Ferrarese, S.; Beghi, C.; De Ponti, R. Catheter ablation of atrial tachycardias after mitral valve surgery: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 2632–2641.
- Marazzato, J.; Cappabianca, G.; Angeli, F.; Crippa, M.; Golino, M.; Ferrarese, S.; Beghi, C.; De Ponti, R. Ablation of atrial tachycardia in the setting of prior mitral valve surgery. Minerva Cardiol. Angiol. 2021, 69, 94–101.
- Morady, F.; Oral, H.; Chugh, A. Diagnosis and ablation of atypical atrial tachycardia and flutter complicating atrial fibrillation ablation. Heart Rhythm 2009, 6 (Suppl. 8), S29–S32.
- Akerström, F.; Bastani, H.; Insulander, P.; Schwieler, J.; Arias, M.A.; Jensen-Urstad, M. Comparison of Regular Atrial Tachycardia Incidence After Circumferential Radiofrequency versus Cryoballoon Pulmonary Vein Isolation in Real-Life Practice. J. Cardiovasc. Electrophysiol. 2014, 25, 948–952.
- Ciconte, G.; Baltogiannis, G.; De Asmundis, C.; Sieira, J.; Conte, G.; Di Giovanni, G.; Saitoh, Y.; Irfan, G.; Mugnai, G.; Hunuk, B.; et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace 2015, 17, 559–565.
- Wasmer, K.; Mönnig, G.; Bittner, A.; Dechering, D.; Zellerhoff, S.; Milberg, P.; Köbe, J.; Eckardt, L. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 2012, 9, 1660–1666.
- Leitz, P.; Wasmer, K.; Andresen, C.; Güner, F.; Köbe, J.; Rath, B.; Reinke, F.; Wolfes, J.; Lange, P.S.; Ellermann, C.; et al. The Incidence, Electrophysiological Characteristics and Ablation Outcome of Left Atrial Tachycardias after Pulmonary Vein Isolation Using Three Different Ablation Technologies. J. Cardiovasc. Dev. Dis. 2022, 9, 50.
- Pappone, C.; Oreto, G.; Rosanio, S.; Vicedomini, G.; Tocchi, M.; Gugliotta, F.; Salvati, A.; Dicandia, C.; Calabrò, M.P.; Mazzone, P.; et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: Efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001, 104, 2539–2544.
- Satomi, K.; Bänsch, D.; Tilz, R.; Chun, J.; Ernst, S.; Antz, M.; Greten, H.; Kuck, K.H.; Ouyang, F. Left atrial and pulmonary vein macroreentrant tachycardia associated with double conduction gaps: A novel type of man-made tachycardia after circumferential pulmonary vein isolation. Heart Rhythm 2008, 5, 43–51.
- Gerstenfeld, E.P.; Callans, D.J.; Dixit, S.; Russo, A.M.; Nayak, H.; Lin, D.; Pulliam, W.; Siddique, S.; Marchlinski, F. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 2004, 110, 1351–1357.
- Luther, V.; Sikkel, M.; Bennett, N.; Guerrero, F.; Leong, K.; Qureshi, N.; Ng, F.S.; Hayat, S.A.; Sohaib, S.A.; Malcolme-Lawes, L.; et al. Visualizing Localized Reentry with Ultra-High Density Mapping in Iatrogenic Atrial Tachycardia. Circ. Arrhythmia Electrophysiol. 2017, 10, e004724.
- Frontera, A.; Mahajan, R.; Dallet, C.; Vlachos, K.; Kitamura, T.; Takigawa, M.; Cheniti, G.; Martin, C.; Duchateau, J.; Lam, A.; et al. Characterizing localized reentry with high-resolution mapping: Evidence for multiple slow conducting isthmuses within the circuit. Heart Rhythm 2019, 16, 679–685.
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444.
- Tai, C.-T.; Huang, J.-L.; Lin, Y.-K.; Hsieh, M.-H.; Lee, P.-C.; Ding, Y.-A.; Chang, M.-S.; Chen, S.-A. Noncontact three-dimensional mapping and ablation of upper loop re-entry originating in the right atrium. J. Am. Coll. Cardiol. 2002, 40, 746–753.
- Stevenson, I.H.; Kistler, P.M.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Morton, J.B.; Kalman, J.M. Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: Electroanatomic characterization and ablation outcome. Heart Rhythm 2005, 2, 594–601.
- Marrouche, N.F.; Natale, A.; Wazni, O.M.; Cheng, J.; Yang, Y.; Pollack, H.; Verma, A.; Ursell, P.; Scheinman, M.M. Left septal atrial flutter: Electrophysiology, anatomy, and results of ablation. Circulation 2004, 109, 2440–2447.
- Kharbanda, R.K.; Özdemir, E.H.; Taverne, Y.J.; Kik, C.; Bogers, A.J.; de Groot, N.M. Current Concepts of Anatomy, Electrophysiology, and Therapeutic Implications of the Interatrial Septum. JACC Clin. Electrophysiol. 2019, 5, 647–656.
- Baker, B.M.; Lindsay, B.D.; Bromberg, B.I.; Frazier, D.W.; Cain, M.E.; Smith, J.M. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: Localizing and transecting the critical isthmus. J. Am. Coll. Cardiol. 1996, 28, 411–417.
- Triedman, J.K.; Bergau, D.M.; Saul, J.; Epstein, M.R.; Walsh, E.P. Efficacy of Radiofrequency Ablation for Control of Intraatrial Reentrant Tachycardia in Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 1997, 30, 1032–1038.
- Jaïs, P.; Shah, D.C.; Haïssaguerre, M.; Hocini, M.; Peng, J.T.; Takahashi, A.; Garrigue, S.; Le Métayer, P.; Clémenty, J. Mapping and ablation of left atrial flutters. Circulation 2000, 101, 2928–2934.
- Magnin-Poull, I.; De Chillou, C.; Miljoen, H.; Andronache, M.; Aliot, E. Mechanisms of Right Atrial Tachycardia Occurring Late After Surgical Closure of Atrial Septal Defects. J. Cardiovasc. Electrophysiol. 2005, 16, 681–687.
- De Ponti, R.; Verlato, R.; Bertaglia, E.; Del Greco, M.; Fusco, A.; Bottoni, N.; Drago, F.; Sciarra, L.; Ometto, R.; Mantovan, R.; et al. Treatment of macro-re-entrant atrial tachycardia based on electroanatomic mapping: Identification and ablation of the mid-diastolic isthmus. Europace 2007, 9, 449–457.
- Vlachos, K.; Efremidis, M.; Derval, N.; Martin, C.A.; Takigawa, M.; Bazoukis, G.; Frontera, A.; Gkalapis, C.; Duchateau, J.; Nakashima, T.; et al. Use of high-density activation and voltage mapping in combination with entrainment to delineate gap-related atrial tachycardias post atrial fibrillation ablation. Europace 2021, 23, 1052–1062.
- Esato, M.; Hindricks, G.; Sommer, P.; Arya, A.; Gaspar, T.; Bode, K.; Bollmann, A.; Wetzel, U.; Hilbert, S.; Kircher, S.; et al. Color-coded three-dimensional entrainment mapping for analysis and treatment of atrial macroreentrant tachycardia. Heart Rhythm 2009, 6, 349–358.
- Grubb, C.S.; Lewis, M.; Whang, W.; Biviano, A.; Hickey, K.; Rosenbaum, M.; Garan, H. Catheter Ablation for Atrial Tachycardia in Adults with Congenital Heart Disease: Electrophysiological Predictors of Acute Procedural Success and Post-Procedure Atrial Tachycardia Recurrence. JACC Clin. Electrophysiol. 2019, 5, 438–447.
- Liu, S.; Lin, Y.; Lee, P.; Vicera, J.J.; Chang, S.; Lo, L.; Hu, Y.; Chung, F.; Tuan, T.; Chao, T.; et al. The isthmus characteristics of scar-related macroreentrant atrial tachycardia in patients with and without cardiac surgery. J. Cardiovasc. Electrophysiol. 2021, 32, 1921–1930.
- Delacretaz, E.; Ganz, L.I.; Soejima, K.; Friedman, P.L.; Walsh, E.P.; Triedman, J.K.; Sloss, L.J.; Landzberg, M.J.; Stevenson, W.G. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: Entrainment mapping combined with three-dimensional electroanatomic mapping. J. Am. Coll. Cardiol. 2001, 37, 1665–1676.
- Zhang, J.; Tang, C.; Zhang, Y.; Han, H.; Li, Z.; Su, X. Electroanatomic Characterization and Ablation Outcome of Nonlesion Related Left Atrial Macroreentrant Tachycardia in Patients without Obvious Structural Heart Disease. J. Cardiovasc. Electrophysiol. 2012, 24, 53–59.
- Chae, S.; Oral, H.; Good, E.; Dey, S.; Wimmer, A.; Crawford, T.; Wells, D.; Sarrazin, J.-F.; Chalfoun, N.; Kühne, M.; et al. Atrial Tachycardia After Circumferential Pulmonary Vein Ablation of Atrial Fibrillation: Mechanistic Insights, Results of Catheter Ablation, and Risk Factors for Recurrence. J. Am. Coll. Cardiol. 2007, 50, 1781–1787.
- Anter, E.; McElderry, T.H.; Contreras-Valdes, F.M.; Li, J.; Tung, P.; Leshem, E.; Haffajee, C.I.; Nakagawa, H.; Josephson, M.E. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm 2016, 13, 2048–2055.
- Balt, J.C.; Klaver, M.N.; Mahmoodi, B.K.; van Dijk, V.F.; Wijffels, M.C.E.F.; Boersma, L.V.A. High-density versus low-density mapping in ablation of atypical atrial flutter. J. Interv. Card. Electrophysiol. 2021, 62, 587–599.
- De Ponti, R.; Marazzi, R.; Zoli, L.; Caravati, F.; Ghiringhelli, S.; Salerno-Uriarte, J.A. Electroanatomic mapping and ablation of macroreentrant atrial tachycardia: Comparison between successfully and unsuccessfully treated cases. J. Cardiovasc. Electrophysiol. 2010, 21, 155–162.
- Drago, F.; Russo, M.S.; Marazzi, R.; Salerno-Uriarte, J.A.; Silvetti, M.S.; De Ponti, R. Atrial tachycardias in patients with congenital heart disease: A minimally invasive simplified approach in the use of three-dimensional electroanatomic mapping. Europace 2011, 13, 689–695.
- Maheshwari, A.; Shirai, Y.; Hyman, M.C.; Arkles, J.S.; Santangeli, P.; Schaller, R.D.; Supple, G.E.; Nazarian, S.; Lin, D.; Dixit, S.; et al. Septal Versus Lateral Mitral Isthmus Ablation for Treatment of Mitral Annular Flutter. JACC Clin. Electrophysiol. 2019, 5, 1292–1299.
- Ouyang, F.; Ernst, S.; Vogtmann, T.; Goya, M.; Volkmer, M.; Schaumann, A.; Bänsch, D.; Antz, M.; Kuck, K.H. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: Critical isthmus block can prevent atrial tachycardia recurrence. Circulation 2002, 105, 1934–1942.
- Tanner, H.; Lukac, P.; Schwick, N.; Fuhrer, J.; Pedersen, A.K.; Jansen, P.S.; Delacretaz, E. Irrigated-tip catheter ablation of intraatrial reentrant tachycardia in patients late after surgery of congenital heart disease. Heart Rhythm 2004, 1, 268–275.
- Nakagawa, H.; Shah, N.; Matsudaira, K.; Overholt, E.; Chandrasekaran, K.; Beckman, K.J.; Spector, P.; Calame, J.D.; Rao, A.; Hasdemir, C.; et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal″ ablation. Circulation 2001, 103, 699–709.
- Bogun, F.; Bender, B.; Li, Y.-G.; Hohnloser, S.H. Ablation of atypical atrial flutter guided by the use of concealed entrainment in patients without prior cardiac surgery. J. Cardiovasc. Electrophysiol. 2000, 11, 136–145.
- Sroubek, J.; Rottmann, M.; Barkagan, M.; Leshem, E.; Shapira-Daniels, A.; Brem, E.; Bs, C.F.; Bs, J.M.; Basu, S.; Bar-Tal, M.; et al. A novel octaray multielectrode catheter for high-resolution atrial mapping: Electrogram characterization and utility for mapping ablation gaps. J. Cardiovasc. Electrophysiol. 2019, 30, 749–757.
- Sarkozy, A.; Vijgen, J.; De Potter, T.; Schilling, R.; Markides, V. An early multicenter experience of the novel high-density star-shaped mapping catheter in complex arrhythmias. J. Interv. Card. Electrophysiol. 2022, in press.
- Rillo, M.; Palamà, Z.; Punzi, R.; Vitanza, S.; Aloisio, A.; Polini, S.; Tucci, A.; Msc, A.P.; Zonno, F.; Anastasia, A.; et al. A new interpretation of nonpulmonary vein substrates of the left atrium in patients with atrial fibrillation. J. Arrhythmia 2021, 37, 338–347.
- Seiler, J.; Schmid, D.K.; Irtel, T.A.; Tanner, H.; Rotter, M.; Schwick, N.; Delacrétaz, E. Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart 2007, 93, 325–330.
- Ho, S.Y.; Anderson, R.H.; Sanchez-Quintana, D. Atrial structure and fibres: Morphologic bases of atrial conduction. Cardiovasc. Res. 2002, 54, 325–336.
- Pambrun, T.; Duchateau, J.; Delgove, A.; Denis, A.; Constantin, M.; Ramirez, F.D.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; et al. Epicardial course of the septopulmonary bundle: Anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021, 18, 349–357.
