The COVID-19 pandemic caused by SARS-CoV-2 remains a significant issue for global health, the economy, and society. When SARS-CoV-2 began to spread, the most recent serious infectious disease of this century around the world, with its high morbidity and mortality rates, it is understandable why such infections have generally been spread in the past, mainly from international travel movements. Microbiology is a branch of medicine and biology that studies the structure and functions of microorganisms (i.e., all those single-celled, multicellular or acellular living organisms not visible to the naked eye such as bacteria, Archaea, some types of fungi, algae, protozoa, viruses and prions).
- SARS-CoV-2
- human vaccines
- COVID-19
- pandemic
- Pubblic health
- Epidemiology
- Infections
- Viruses
- Health care management
1. Diagnostic Tests and Screening
2. Vaccination Campaign
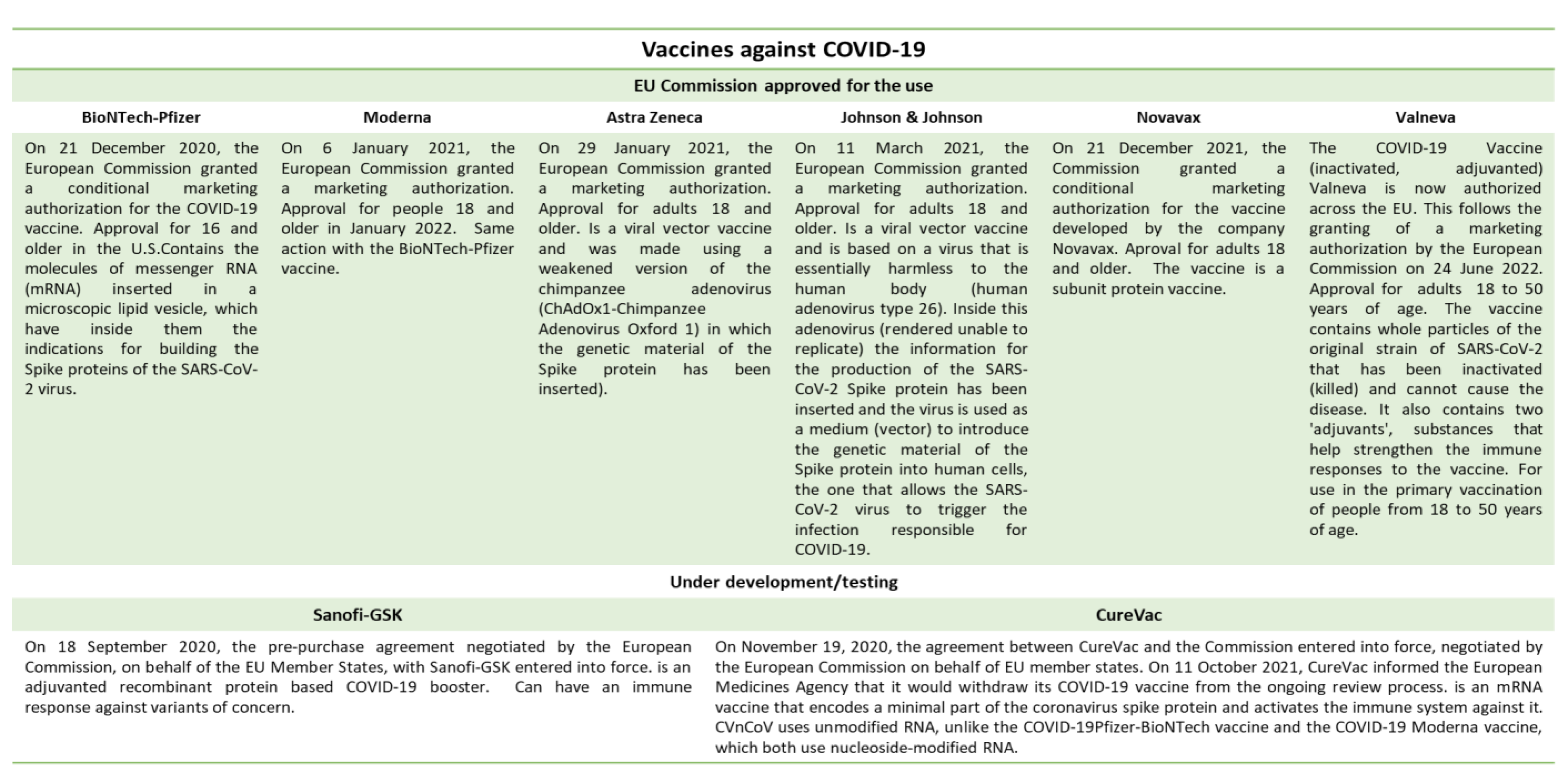
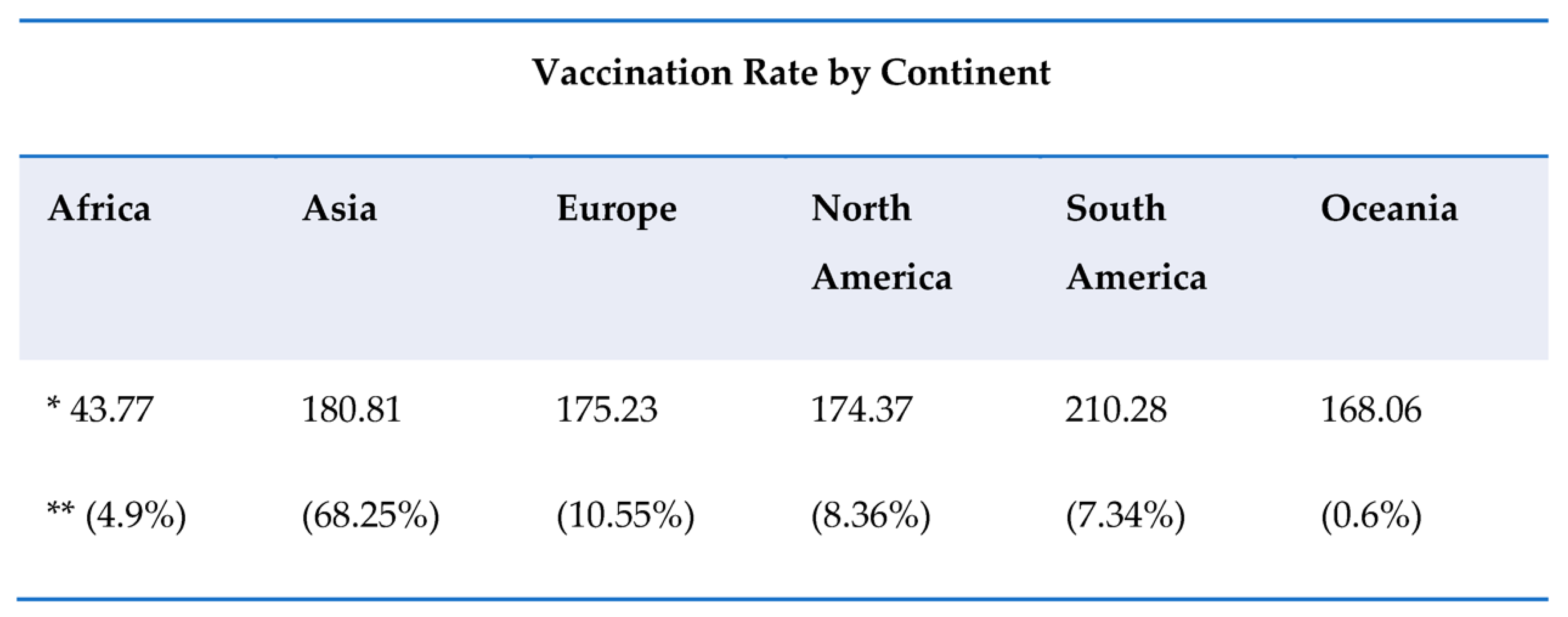
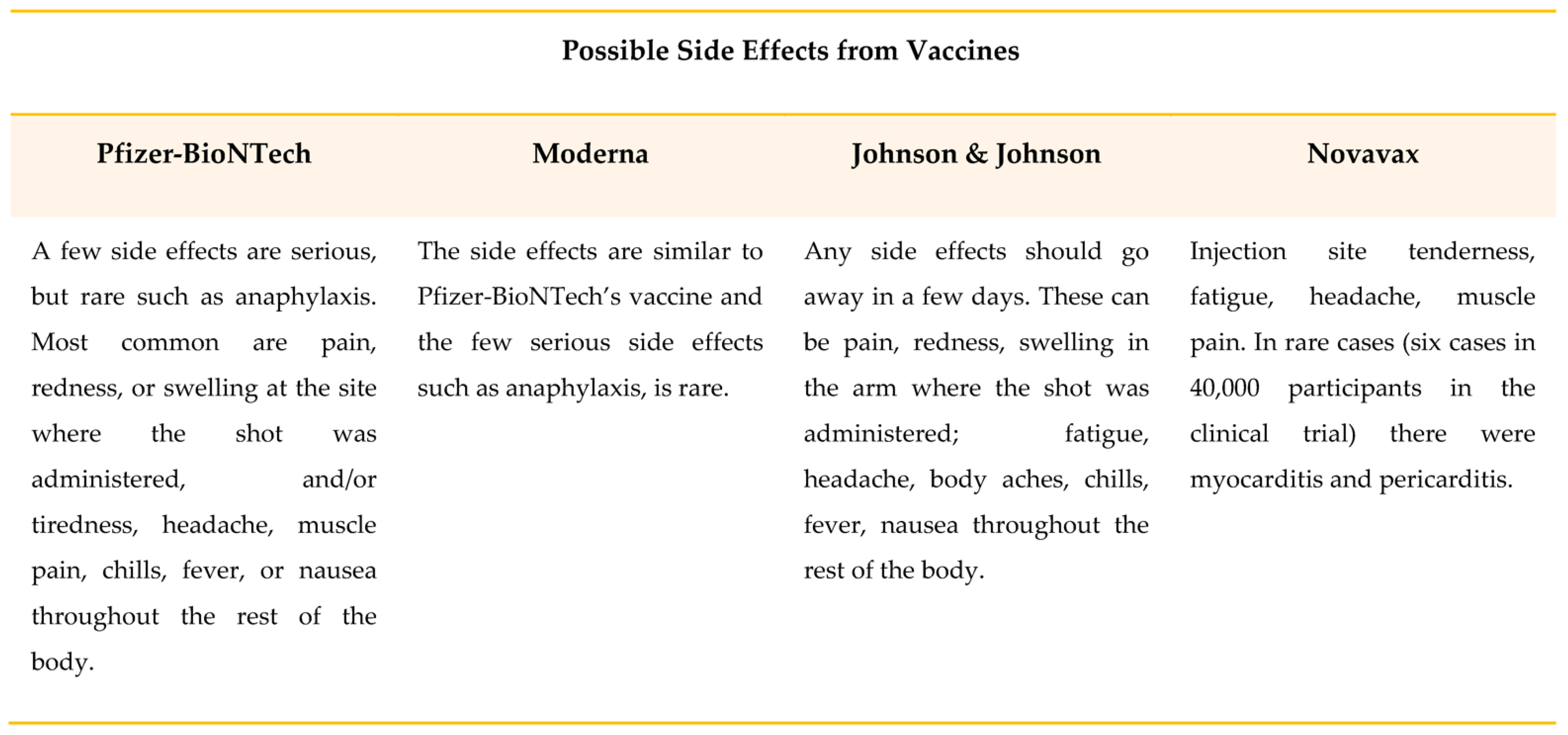
This entry is adapted from the peer-reviewed paper 10.3390/ijerph191710712
References
- WHO. Coronavirus Disease (COVID-19): Weekly Epidemiological Update (7 December 2021). Available online: https://reliefweb.int/report/world/coronavirus-disease-covid-19-weekly-epidemiological-update-7-december-2021 (accessed on 29 July 2022).
- Charitos, I.A.; Del Prete, R.; Inchingolo, F.; Mosca, A.; Carretta, D.; Ballini, A.; Santacroce, L. What we have learned for the future about COVID-19 and healthcare management of it? Acta Biomed. 2020, 91, e2020126.
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241.
- Schirinzi, A.; Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Ciavarella, D.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Serio, F.; et al. New Insights in Laboratory Testing for COVID-19 Patients: Looking for the Role and Predictive Value of Human epididymis secretory protein 4 (HE4) and the Innate Immunity of the Oral Cavity and Respiratory Tract. Microorganisms 2020, 8, 1718.
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential beneficial role of probiotics on the outcome of COVID-19 patients: An evolving perspective. Diabetes Metab. Syndr. 2021, 15, 295–301.
- Sachdeva, K.; Kumar, A.; Mohanty, S. Virology of SARS-CoV-2 and management of nCOVID-19 utilizing immunomodulation properties of human mesenchymal stem cells-a literature review. Stem Cell Investig. 2021, 8, 23.
- Arabpour, E.; Khoshdel, S.; Tabatabaie, N.; Akhgarzad, A.; Zangiabadian, M.; Nasiri, M.J. Stem Cells Therapy for COVID-19: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 737590.
- Kolanko, E.; Mazurski, A.; Czekaj, P. Potential therapeutic application of mesenchymal stem cells in COVID-19 complications. Med. Pr. 2021, 72, 693–700.
- Sanie-Jahromi, F.; NejatyJahromy, Y.; Jahromi, R.R. A Review on the Role of Stem Cells against SARS-CoV-2 in Children and Pregnant Women. Int. J. Mol. Sci. 2021, 22, 11787.
- Sampson, V. Could Poor Oral Hygiene Increase the Risk of COVID-19 Infection? Compend. Contin. Educ. Dent. 2021, 42, 144.
- Segrelles-Calvo, G.; de SAraújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Med. 2021, 188, 106619.
- Brikman, S.; Dori, G.; Kasher, C.; Yanovskay, A.; Strauss, M.; Colodner, R.; Bisharat, N.; Chazan, B. Candida Bloodstream Infection, a Dire Complication in Hospitalized COVID-19 Patients: Three Cases from a Single Center in Northern Israel. Isr. Med. Assoc. J. 2021, 23, 615–617.
- Ohashi, N.; Ideta, Y.; Takeda, A.; Iwai, T.; Kioi, M.; Miyazaki, A.; Mitsudo, K. Oral candidiasis caused by ciclesonide in a patient with COVID-19 pneumonia: A case report and literature review. SAGE Open Med. Case Rep. 2021, 9, 2050313X211048279.
- Brody, R.M.; Albergotti, W.G.; Shimunov, D.; Nicolli, E.; Patel, U.A.; Harris, B.N.; Bur, A.M. Changes in head and neck oncologic practice during the COVID-19 pandemic. Head Neck. 2020, 42, 1448–1453.
- European Centre for Disease Prevention and Control (ECDC). How to Protect Yourself and Others. 2021. Available online: https://www.ecdc.europa.eu/en/covid-19/prevention-and-control/protect-yourself (accessed on 15 July 2022).
- Santacroce, L.; Charitos, I.A.; Carretta, D.M.; de Nitto, E.; Lovero, R. The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. J. Mol. Med. 2021, 99, 93–106.
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J. Oral Sci. 2020, 63, 1–3.
- Borges, L.P.; Martins, A.F.; Silva, B.M.; Dias, B.P.; Gonçalves, R.L.; Souza, D.R.V.; Oliveira, M.G.B.; Jesus, P.C.; Serafini, M.R.; Quintans, J.S.S.; et al. Rapid diagnosis of COVID-19 in the first year of the pandemic: A systematic review. Int. Immunopharmacol. 2021, 101 Pt A, 108144.
- Centers for Disease Control and Prevention (CDC), USA. Interim Guidance for SARS-CoV-2 Testing in Non-Healthcare Workplaces. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/testing-non-healthcare-workplaces.html (accessed on 31 July 2022).
- World Health Organization (WHO). Coronavirus disease (COVID-19): Vaccines. 2021. Available online: https://www.who.int/news/item/11-06-2021-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness (accessed on 20 July 2022).
- Food and Drug Administration (FDA). Screening for COVID-19: Deciding Which Test to Use When Establishing Testing Programs. 2021. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/screening-covid-19-deciding-which-test-use-when-establishing-testing-programs (accessed on 29 July 2022).
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 16 July 2022).
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.; Failla, G.; Ricciardi, W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review. EClinicalMedicine 2021, 40, 101113.
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130433.
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295.
- Ada, G. Overview of vaccines and vaccination. Mol. Biotechnol. 2005, 29, 255–272.
- Santacroce, L.; Bottalico, L.; Charitos, I.A. The Impact of COVID-19 on Italy: A Lesson for the Future. Int. J. Occup. Environ. Med. 2020, 11, 151–152.
- Centers for Disease Control and Prevention (CDC), USA. COVID Data Tracker 2021. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccine-effectivenes (accessed on 18 July 2022).
- World Health Organization (WHO). Vaccine Efficacy, Effectiveness and Protection. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 29 July 2022).
- Centers for Disease Control and Prevention (CDC), USA. Different COVID-19 Vaccines. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html (accessed on 31 July 2022).
- Centers for Disease Control and Prevention (CDC), USA. Pfizer-BioNTech COVID-19 Vaccine. 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/storage-summary.pdfThe (accessed on 30 July 2022).
- EU Vaccination Strategy. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/eu-vaccines-strategy_el#---3 (accessed on 19 July 2022).
- European Medicines Agency (EMA). Recommends Valneva’s COVID-19 Vaccine for Authorisation in the EU (24/06/2022). Available online: https://www.ema.europa.eu/en/news/ema-recommends-valnevas-covid-19-vaccine-authorisation-eu (accessed on 16 July 2022).
- Lindstrand, A.; Cherian, T.; Chang-Blanc, D.; Feikin, D.; O’Brien, K.L. The World of Immunization: Achievements, Challenges, and Strategic Vision for the Next Decade. J. Infect. Dis. 2021, 224 (Suppl. S2), S452–S467.
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513.
- Rella, S.A.; Kulikova, Y.A.; Dermitzakis, E.T.; Kondrashov, F.A. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci. Rep. 2021, 11, 15729.
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394.
- Wang, J.; Lu, X.; Lai, X.; Lyu, Y.; Zhang, H.; Fenghuang, Y.; Jing, R.; Li, L.; Yu, W.; Fang, H. The Changing Acceptance of COVID-19 Vaccination in Different Epidemic Phases in China: A Longitudinal Study. Vaccines 2021, 9, 191.
- World Health Organization (WHO). Coronavirus Disease (COVID-19): Vaccines Safety. 2021. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety (accessed on 29 July 2022).
- Bubar, K.M.; Middleton, C.E.; Bjorkman, K.K.; Parker, R.; Larremore, D.B. SARS-CoV-2 Transmission and Impacts of Unvaccinated-Only Screening in Populations of Mixed Vaccination Status. medRxiv Prepr. Serv. Health Sci. 2022, 19, 2777.
- WHO. Vaccine Equity. Available online: https://www.who.int/campaigns/vaccine-equity (accessed on 29 July 2022).
- WHO. COVID-19 Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 30 July 2022).
- Oxford University. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 31 July 2022).
- European Center for Disease Prevention and Control (ECDC). Preliminary Public Health Considerations for COVID-19 Vaccination Strategies in the Second Half of 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/preliminary-public-health-considerations-covid-19-vaccination-strategies-second (accessed on 16 July 2022).
- Katella, K. Comparing the COVID-19 Vaccines: How Are They Different? Yale Medicine. 2022. Available online: https://www.yalemedicine.org/news/covid-19-vaccine-comparison (accessed on 19 July 2022).
- Li, Y.; Wang, X.; Jin, J.; Ma, Z.; Liu, Y.; Zhang, X.; Su, B. T-cell responses to SARS-CoV-2 Omicron spike epitopes with mutations after the third booster dose of an inactivated vaccine. J. Med. Virol. 2022, 94, 3998–4004.
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193.
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. SARS-CoV-2 Naturally Acquired Im-munity vs. Vaccine-induced Immunity, Reinfections versus Breakthrough Infec-tions: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551.
- Tut, G.; Lancaster, T.; Sylla, P.; Butler, M.S.; Kaur, N.; Spalkova, E.; Bentley, C.; Amin, U.; Jadir, A.; Hulme, S.; et al. Antibody and cellular immune responses following dual COVID-19 vaccination within infection-naive residents of long-term care facilities: An observational cohort study. Lancet Healthy Longev. 2022, 3, e461–e469.
