Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Anillin (ANLN) is an actin-binding protein that has been documented as a key factor in cell division, and it is a multi-domain protein that interacts with many proteins. ANLN is highly expressed in many types of site-specific cancerous tumours, including brain, lung, pancreas, and bone marrow cancer.
- anillin
- cytokinesis
- actin-binding protein
1. Findings from Drosophila and C. elegans
ANLN was isolated as an F-actin-binding and bundling protein from Drosophila melanogaster embryo extracts [12]. ANLN localizes to the nucleus during interphase, to the cortex upon nuclear envelope breakdown, to the cleavage furrow in anaphase and to the midbody rings during telophase and into the next cell cycle [12].
RNAi (RNA interference)-mediated depletion of ANLN causes furrow instability in D. melanogaster S2 cells [13]. In these cells, furrows form normally at the cell equator, but then oscillate back and forth across the equator, parallel to the spindle axis. The loss of ANLN also promotes membrane blebbing and, in cases where a relatively stable furrow forms, a loss of stability of the midbody structure that forms after furrowing in D. melanogaster [14]. In the first division of Caenorhabditis elegans embryos, ANLN depletion does not appear to prevent cytokinesis but causes a loss of furrow asymmetry [15].
ANLN is a highly conserved protein that contains multiple domains [16] (Figure 1). These domains were documented to interact with many partners (Table 1), such as actin, myosin II and septins [1]. ANLN could act as an indispensable factor to scaffold and organize the cytoskeleton and its partners in the events related to cytokinesis [11,12,17]. ANLN is majorly associated with cortical cytoskeletal dynamics during cytokinesis and cellularization. However, other roles in different contexts are possible to be masked by their fundamental functions [11].
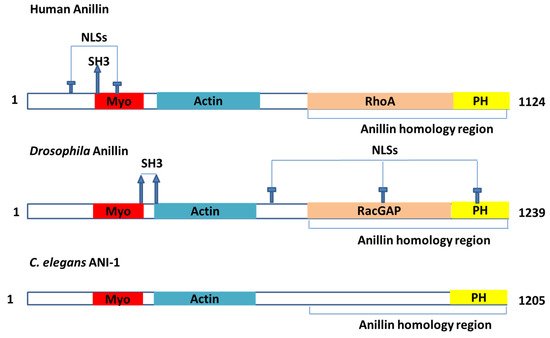
Figure 1. The model of the major domains in the ANLN actin-binding protein. My, myosin-II-binding domain; Actin, actin-binding domain; NLS, nuclear localization signal; PH, pleckstrin homology domain; RacGAP, region that interacts with the RacGAP50C component of the centralspindlin complex; RhoA, region that interacts with the GTPase RhoA; SH3, Src-homology-3-binding consensus sequences.
Table 1. The roles and characteristics of ANLN and its partners in cytokinesis.
| Partners of ANLN | System | Binding Regions of ANLN | Binding Regions of Partners | Ref | Role of ANLN to Partners | Characteristics in the Association between ANLN and the Partners |
|---|---|---|---|---|---|---|
| Actin | Drosophila | Amino acids 258–340 | Full length | [12] |
|
|
| Human | Amino acids 231–454 | Full length | [22] | |||
| X. Leavis | Amino acids 255–418 | Full length | [23] | |||
| Septins (a group of GTP-binding proteins) | Human | Amino acids 929–1125 | Full length | [22] |
|
|
| Human | Amino acid 748–1116 | Full length | [23] | |||
| Drosophila | Amino acids 815–1201 | Full length | [26] | |||
| Myosin | X. Leavis | Amino acids 142–254 | Full length | [27] |
|
|
| RhoA | Human | Amino acids 608–943 | Full length | [16] |
|
|
| RacGap | Drosophila | Amino acids 517–1212 | Amino acids 83–309 | [30] |
|
|
| Drosophila | Amino acids 929–129 | Amino acids 136–371 | [31] | |||
| Ect2 | Human | Amino acids 926–980 | Full length | [1] |
|
|
| Human | Amino acids 421–621 | Amino acids 608–940 | [32] | |||
| CD2A | Human | Amino acids 1–155 | Amino acids 1–175 | [33] |
|
|
| Drosophila | Amino acids 1–328 | Full length | [34] | |||
| Drosophila | Amino acids 930–1239 | Full length | [34] | |||
| Microtubules | Drosophila | Full length | Full length | [35] |
|
|
2. Binding Partners
ANLN, a conserved multi-domain protein interacting with many biological partners, is a prime factor that may act as a scaffold and be involved in organizing the cytoskeleton, as well as be a regulator in the entire aforementioned events.
2.1. Binding Partners Related to Cytokinesis
ANLN interacts with some proteins during cytokinesis (Table 1). Among its partners, there are some proteins proven to be associated with cancer. Take actin, myosin, septins, RhoA, and RacGAP for example.
Actin: Actin is a protein that is abundant in mammalian cells, and it is associated with the motility and compartmentalization of cellular contents. In eukaryotic cells, there are two main actin forms: globular G-actin and fibrillar F-actin. F-actin is composed of G-actin. G-actin can polymerize in the absence of associated proteins after ATP hydrolysis and Mg2+ consumption in vitro. However, in cells, actin polymerization is accelerated by actin nucleation factors such as Arp2/3 and formins [36]. The growth of these actin filaments is regulated by thymosin and profilin. Thymosin binds to G-actin to buffer the polymerizing process, while profilin binds to G-actin to exchange ADP for ATP, promoting the monomeric addition to the barbed, plus end of F-actin filaments [36,37]. Filaments are assembled and structured by actin-filament-bundling proteins [38]. ANLN was characterized as a molecule that is specifically associated with F-actin [18]. ANLN interacts with three types of actin filaments [12,23,39]. ANLN bundles actin filaments with two domains, including the amino acids 258–340 of Drosophila. ANLN was also described as a binding site for F-actin and amino acids 246–371 bundle actin filaments. The F-actin-binding domain of ANLN was recorded with two F-actin-binding sites, but one of them could shrink after forming a binding with F-actin [12]. F-actin and ANLN are independently attracted to the contractile ring in human and Drosophila cells [13,19,20,21].
Myosin: ANLN could form an interaction with myosin, directly or indirectly [1]. ANLN may indirectly impact myosin through F-actin [1]. There has been evidence showing that in Drosophila and C. elegans, ANLN (ANI-1 in C. elegans) and myosin are attracted to the contractile ring independently [13,25,31]. The main function of ANLN to myosin is to organize them. This was observed in a finding of Haglund K in 2010, which found that ANLN in Drosophila is necessary and sufficient to organize myosin into rings during cellularization [41]. During cytokinesis, there is a disruption of the stability of timing and space at the cell equator of ANLN-depleted cells (human and Drosophila) [13,16,19,21,31,42]. C. elegans ANLN was documented to be involved in the organization of myosin into dynamic foci within the span of the polarity formation and cytokinesis [25,43] and encourages asymmetric furrow ingression located at the zygote [27]. C. elegans ANLN (ANI-2) is required for the integrity of the myosin [25].
Septins: Septins belong to a group of GTP-binding proteins [44,45]. Different septins make a complex with one another [44]. These complexes can assemble into filaments and rings functioning as a fourth cytoskeleton [46]. ANLN was documented as a partner that binds to septins [1,46,47], a conserved family of GTP-binding proteins [48]. Septins are also recruited by ANLN to the contractile ring [24]. There was a direct interaction between the septins and ANLN identified in vitro [23]. The third C-terminal was characterized as a binding part to septins, whereas the third C-terminal was constituted by the terminal PH domain and the ANLN homology (AH) [1]. ANLN truncation occurs in human cells without the AH domain, which was considered as a factor mediating the interaction of septins, and showed the mislocalization of the poles in the span of oscillation like the event witnessed as a lack of myosin [16].
RhoA: RhoA is a protein involved in multiple cellular processes that plays a central role in the regulation of actin organization, cell migration, cytokinesis, cell cycle regulation and cell proliferation [49,50,51,52]. The human AH domain could act as a bridge between ANLN and RhoA [53,54]. ANLN and RhoA were discovered to be co-immunoprecipitated [29]. Furthermore, the upregulation of ANLN could induce a significant increase in the rate of active RhoA [29]. The equatorial cortical localization of RhoA tended to be regulated by ANLN during cytokinesis because ANLN is associated with the cell membrane in both ways of direction or indirection via septins [1]. ANLN and RhoA were documented to have locations in proximity with each other [1]. The AH domain in the C-terminus of ANLN is able to bind directly to RhoA in vitro, and the role of this domain was proven to stabilize RhoA localization in vivo [16]—all of which could indicate that ANLN could be associated with activating RhoA and stabilizing it in the cleavage plane [1]. The interaction between ANLN and Ect2 (an activator of RhoA) was found, which supports the postulation about ANLN involved in regulating and stabilizing RhoA location [1].
RacGAP: ANLN was recorded to interact directly with RacGAP50C [1,30], which plays a role in specifying the cleavage site. This interaction was confirmed by a yeast two-hybrid assay. In another study, RacGAP was discovered to interact with full-length ANLN via amino acids 83–309 while the sequence of amino acids from 245 to 311 of RacGAP plays a role in the interaction with ANLN [30]. The half of the ANLN N-terminal of ANLN was observed not to interact with any RacGAP constructs. Specific RacGAP deletions to abolish Pebble or MKLP1 binding was documented without any effect on the interaction with ANLN [55]. The absence of ANLN leads to a loss of connection between the spindle-associated RacGAP and the equatorial cortex and to cytokinesis failure [30]. ANLN was also documented to interact with [56] and co-express [57] RacGAP1.
2.2. Other Binding Partners
Kinases: ANLN was proved to be phosphorylated in early mitosis [33]. Forty-six phosphorylation sites of ANLN were discovered, but only phosphorylation at S635 was proven as an important requirement for the success of ANLN recruitment to cleavage furrow. This was also underpinned by the evidence of phosphomimetic-mutant S635D, the negative charge of D at the 635 residues which partially recovered the localization. S635 phosphorylation helps ANLN improve the efficacy of the Rho integration with its upstream and downstream regulators, which contributes to the success of cytokinesis [58]. To date, the kinases that are responsible for S635 remain unidentified, so there is a need to determine which kinases are responsible for the phosphorylation at S635.
CDK1 was found to interact with ANLN [56,57], and it is indicated that ANLN mobility is directly or indirectly regulated by CDK1 via phosphorylation [58]. The ANLN-actin-binding protein has been identified as being involved in PI3K/PTEN signaling, which is critical in cell life/death control [59]. CITK was proven to be associated with the localization of F-actin and ANLN at the abscission sites [60].
KIAA1429: KIAA1429, a complex that is involved in the regulation of the N6-methyladenosine (m6A) methylation of RNAs, is a modification that plays a role in the effectiveness of mRNA splicing and RNA processing [61]. The interaction between KIAA1429 and ANLN was found by methods including affinity capture-mass spectrometry (MS) and affinity capture-RNA [62], and it is curated by BIOGRID [63]. KIAA1429 has recently been recognized as an oncogenic factor in several cancer types, including breast cancer, by regulating CDK1 [64]. KIAA1429 was also found to be associated with the migration and invasion of hepatocellular carcinoma by altering the m6A modification of ID2 mRNA [65]. The contribution of KIAA1429 to liver cancer progression was recorded [66]. The interaction between KIAA1429 and ANLN could suggest that we could monitor KIAA1429 expression through ANLN.
MYC: Myc is a well known family of regulator genes and proto-oncogenes that code for transcription factors. The Myc family includes three related human genes: c-Myc, l-Myc, and n-Myc. With a possible role in cancer, c-Myc is often expressed. The protein encoded by the Myc gene could act as a multifunctional protein that could be associated with cell growth, cellular transformation, and apoptosis [67]. The upregulation of Myc could be present in various types of cancers, such as colon, breast, lung and gastric cancer [68]. To date, Myc and ANLN have been discovered to interact through experimental evidence, including affinity capture-MS [69], and proximity label-MS [70] and curated by the Biogrid [63].
KDR: Kinase insert domain receptor (KDR) is a primary vascular endothelial growth factor receptor that encodes a crucial receptor regulating the cancer angiogenesis/metastasis switch [59]. KDR is also a key factor in controlling the survival, growth and migration of endothelial cells. Its upregulation has been found in various types of cancer cells [71]. ANLN has been identified as an interactor with KDR that encodes a key receptor mediating the cancer angiogenesis/metastasis switch. The observations have suggested that ANLN acts as the intrinsic connection between PI3K/PTEN and KDR signaling, which represents two critical transitions in carcinogenesis [59]. The interaction between ANLN and KDR could act as ANLN and KDR jointly as a prognostic in cancer survival, which could be applied to control triple negative breast cancer [71].
CDC5L: CDC5L is a DNA-binding protein that is implicated in cell cycle control and could act as a transcription factor. It takes a role in pre-mRNA splicing as a core factor of precatalytic, catalytic and post catalytic spliceosomal complexes [72,73,74,75,76,77,78,79]. The depletion of CDC5L leads to the inhibition of mitotic progression, and it induces mitotic failure [80]. Additionally, the downregulation of CDC5l inhibits the proliferation of bladder cancer cells [81]. ANLN has been experimentally proven to interact with CDC5L [82]. CDC5L is also located in the nucleus [83] where ANLN is primarily present during the cell cycle. In this regard, ANLN is likely to play a role in the splicing involving CDC5L.
TAF10: Gene ontology annotations associated with the TATA-box-binding protein associated factor 10 (TAF10) are the DNA-binding transcription factor activity and the transcription coactivator activity. TAF10 is involved in the process of Drosophila erythropoiesis via the GATA1 transcription factor [84], and it appears to play a dispensable role in the somitogenesis process and the Drosophila morphogenesis process in mice [85,86]. TAF10 inactivation in liver tissue was observed to dissociate TFIID complexes individually, but genes affected by TAF10 inactivation were less than 5% of the active genes [87]. ANLN interacts with TAF10 [88] and is present in the nucleus when it is in interphase cells and syncytial embryo [12]. It seems that ANLN is likely to function in relation to the role of TAF10.
BRCA1: BRCA1 is essentially involved in the repair of double-strand breaks by recruiting DNA repair enzymes. Loss of function mutations decrease the repair of DNA double-strand breaks and thereby increases the mutation frequency and the risk of cancer [89]. The encoded protein combines with other tumour suppressors, DNA damage sensors, and signal transducers to form a large multi-subunit protein complex known as the BRCA1-associated genome surveillance complex (BASC) [90]. The BRCA1 mutation is found in breast cancer in young women, which is a triple negative breast cancer [91]. ANLN interacts with BRCA1 [92]. Therefore, there is a possibility that ANLN modulates DNA repair by interacting with BRCA1 in the nucleus.
Others including microtubules: Drosophila ANLN has an affinity with microtubules [26]. The alignment of spindle-microtubules in metaphase was regulated by microtubule ANLN interaction [1]. In addition, human ANLN was documented to interact with astral microtubules [93]. Astral microtubules and spindle microtubules may independently restrict the localization of ANLN and other contractile ring proteins at the equatorial cortex. This sequestration could change the organization of cortical proteins to polarize cells in cytokinesis [93]. Some other potential interactors with ANLN were discovered and curated by String and Biogrid, such as CALML3, KIF 23, KIF20A [57,63].
3. Role of ANLN during Cytokinensis
During mitosis, ANLN locations shift drastically (Figure 2). In vivo immune-staining observations of cultured Drosophila cells and human cells showed that ANLN resides in nuclei during the interphase and relocates to contractile rings during cytokinesis [12,22,39,42].
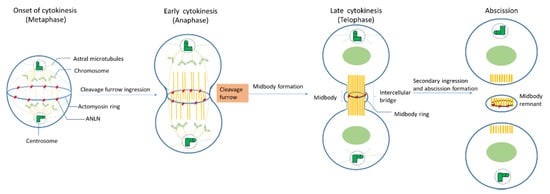
Figure 2. The roles of ANLN during cytokinesis.
Onset of cytokinesis (metaphase): When nuclear envelopes are broken down, ANLN moves from the nuclei to peripheral stress fibers in mammalian cells [23], at which ANLN might mediate these fibers’ disassembly and increase the round cortex of Drosophila cells [13,30]. In S. pombe, Mid1 (an ANLN-like protein) locates at the equatorial cortex before any other contractile ring component and acts as a scaffold for other components such as RhoA, actin, myosin, and the septins [1]. ANLN depletion leads to a mislocalization of F-actin, like myosin, out of the equator in Drosophila spermatocytes, which could lead to a failure in cytokinesis [19].
Early cytokinesis (anaphase): At the beginning of anaphase, the mitotic spindle forms a dense array of antiparallel microtubules called the central spindle [1]. The ingression of the plasma membrane at the cell equator occurs and a cleavage furrow is formed. Although actin–myosin contractility still happens in both human and Drosophila cultured cells that have been depleted of ANLN, the depletion of ANLN leads to the lateral oscillation of cleavage furrow, or its failure cannot accurately maintain at the division plane [13,19,27,31,42].
Late cytokinesis (telophase): At late cytokinesis, the actin and myosin contractile ring that separates daughter cells may assemble through interaction with at least two other furrow proteins, actin, and septins [22]. ANLN-depleted Drosophila cells unveiled that ANLN could function in the stabilization of the midbody because the depletion of ANLN in cells could lead to a reduction in microtubule integrity in the midzone and to blebbing around the midbody which is the central region of the thin intercellular cytoplasmic bridge formed between daughter cells during cytokinesis. It consists of tightly bundled antiparallel microtubules, which embraces a phase-dense circular structure, called the midbody ring [14,94].
Abscission: Before abscission, ANLN and F-actin were maintained at secondary ingression by abscission regulator citron kinase (CITK), and the F-actin localization at secondary ingression sites is required for normal abscission [60], which indicates that ANLN may be relevant for this function. At the final stage of cytokinesis, the intercellular bridge is cleaved in a process termed abscission, and two daughter cells are formed. Following abscission, the residual midbody structure, known as the midbody remnant or midbody derivative, can have different fates depending on the cell type. It can either be released into the extracellular medium, be degraded by autophagy, or persist in the cytoplasm, showing asymmetric accumulation in the daughter cells [36].
4. Mechanism of ANLN-Controlled Cytoskeletal Dynamics
ANLN binds to F-actin: ANLN binds to F-actin only during the cell division process. ANLN forms a bundle of F-actin filaments [12]. By controlling actin bundling, ANLN increases the efficiency of actomyosin contractility during cell division. ANLN and F-actin are independently recruited as contractile rings, but F-actin increases the efficiency of ANLN summons [1]. ANLN also promotes the polymerization of F-actin through stabilizing formin mDia2 in active form. [40].
Regulation of actin–myosin contractility by ANLN: Assembly and contraction of actomyosin filaments promotes cleavage furrow formation and ingression during cytokinesis. The initiation of this furrow is caused by the activation of RhoA by Pebble (Pbl) in Drosophila and RhoGEF, called ECT2 in mammals. Upon the onset of anaphase, the RhoGEF ECT2 is dephosphorylated, which allows it to bind to the centralspindlin. RhoGEF ECT2–centralspindlin binds to ANLN to form a complex. This complex results in the activation of RhoA, which accumulates at the furrow. Active Rho activates several effectors. Active Rho-bound formin induces actin polymerization (F-actin) and the formation of the contractile ring. Active RhoA binds to RBD (RhoA-binding domain) of inactive ROCK and activates ROCK. Active ROCK phosphorylates the regulatory myosin light chain of myosin and the myosin-binding site of myosin phosphatase to activate myosin for furrow ingression. In this manner, these events promote the sliding of myosin heads along the actin filaments, and therefore, the formation and ingression of the cleavage furrow. When contractility force reaches a threshold, p190 RhoGAP-A effectively binds to ANLN at the cytokinesis furrow and turns RhoA-GTP into RhoA-GDP. The reduction of RhoA-GTP leads to a decrease in myosin II activation and F-actin. Finally, the release of p190 RhoGAP-A from ANLN completes one cycle. This mechanism maintains the appropriate contractility force on the actin–myosin ring for the completion of cytokinesis (Figure 3).
Coupling of F-actin to microtubule by ANLN via interaction with RacGAP: In order to activate Pbl/ECT2, which is involved in the activation of RhoA, this molecule requires interaction with RacGAP50C in Drosophila and MgcRacGAP in mammals [96]. This RacGAP molecule is one of two constituent molecules of an evolutionarily conserved complex called centralspindlin [97]. Another constituent molecule of centralspindlin is ZEN-4 in C. elegans, Pavarotti (Pav–KLP) in Drosophila and MKLP1 in mammals called a plus-end-directed kinesin-like motor protein [96].
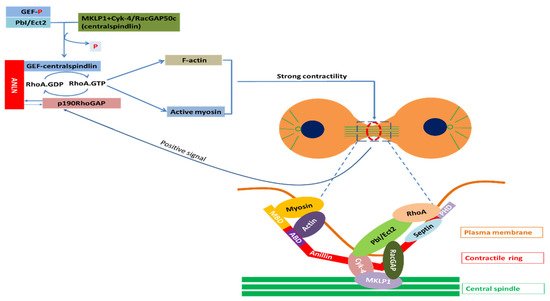
Figure 3. Partners of ANLN in cytokinesis; Myosin, a superfamily of motor proteins; Actin, a family of globular multi-functional proteins that form microfilaments; RhoA, a small GTPase protein in the Rho family of GTPases; Septin, a group of GTP-binding proteins; Cyk-4, a Rho family GTPase-activating protein (Gap) required for central spindle formation and cytokinesis; MKLP1, a member of the kinesin-like protein family; RhoA activation: Ect2, epithelial cell transforming 2 in mammalian cells or PbI in Drosophila; ANLN, anillin actin-binding protein; MBD, myosin-binding domain; ABD, actin-binding domain; PHD, pleckstrin homology domain; GEF, guanine nucleotide exchange factors; p190RhoGAP, Rho GTPase-activating proteins; RacGAP, Rac GTPase-activating protein [96,99].
4.1. ANLN in Nucleus
ANLN is a contractile ring protein that cycles from the nucleus to the cell cortex via importin [12,100]. Although ANLN resides in the nucleus for a relatively long time during a cell cycle, as of now, the roles of ANLN in the nucleus have to date not been determined. Evidence has shown an association between poor tumour prognosis and highly expressed ANLN in the nucleus [8,29,101]. In addition, the downregulation of phosphoinositide 3-kinase/AKT activity in non-small cell lung cancer cells leads to the instability of ANLN and induces a reduction in the ANLN level in the nucleus [29]. This suggests that ANLN can have a role in the nucleus related to the phosphoinositide 3-kinase/AKT pathway.
Among other actin-binding proteins present in the nucleus, FLi1 homolog, α-actinin 4, and filamin A are associated with SWI/SNF, estrogen receptor α, and BRCA 1, 2, respectively, and are involved in chromatin remodeling, transcription regulation, and DNA damage repair, respectively (Figure 4) [83]. ANLN is also known to bind transcription factors such as TAF10, Myc, and BRCA1, so ANLN is likely to be involved in chromatin organization, transcription, and DNA damage repair in the nucleus, respectively [70,92]. Recent reports found that the regulation of actin polymerization in the nucleus is required for transcription activation, cell cycle progression and DNA repair [106,107]. The role of ANLN, an actin-binding protein, has yet to be determined, but it is likely to play a role in the regulation of actin monomer or polymerization processes in the nuclei.
4.2. ANLN in Cytosol
Recently, roles other than the cell division of ANLN have been reported. That is, ANLN regulates adhesion and intercellular junction. For example, mutations in the ANLN gene cause kidney disease and focal segmental glomerulosclerosis, which indicate a defect in podosomal matrix adhesions [10]. Moreover, ANLN is a modulator of cellular cell adhesion mediated by E-cadherin in Drosophila [108]. ANLN knockdown results in abnormal adherens junctions and tight junctions in Xenopus embryos [109].
ANLN is highly expressed at the Z discs of myocardial cells, in which myosin and actin could be found as they anchored tenaciously [3]. In addition, ANLN is associated with preserving the integrity of the podocyte actin cytoskeleton [10]. Besides, ANLN has been recruited to the leading edge of migrating neuroblasts through the activation of MIG-2, a member of the Rho family of small GTPases [110]. Here, it binds active MIG-2 and stabilizes F-actin. The stabilization of F-actin requires ANLN’s ability to prohibit both actin monomer dissociation and the F-actin severing activity of cofilin. Thus, ANLN may be important for proper neuroblast migration and neuritogenesis [110].
It has also been reported to regulate breast cancer cell migration and invasion [113]. However, in this case, it seems that ANLN is present in the nucleus, which controls the movement and invasion of breast cancer cells through JNK signaling. A similar example appears to modulate the integrity of the adherens junction and the tight junction while present in the nucleus during interphase in human epithelial cancer cells DU145, SK-CO15, and A549 [114].
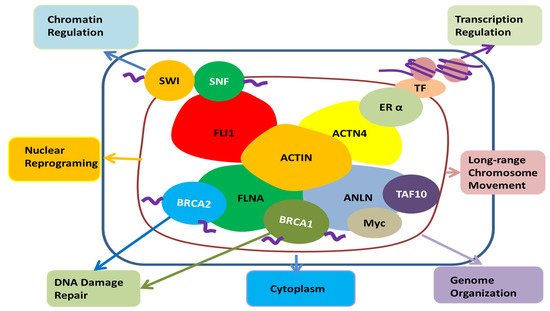
Figure 4. The potential role of ANLN in the nucleus; Actin present in the nucleus is combined with FLi1 homolog, α-actinin 4, filamin A, SWI/SNF, estrogen receptor α, BRCA 1, 2, etc., and it seems to be involved in chromatin remodeling, transcription regulation, nuclear reprogramming, long-range chromosome movement and DNA damage repair. ANLN has the potential to be involved in their action through binding with actin in the nucleus. Moreover, it can be involved in chromatin reorganization, transcription, and DNA damage repair through direct binding with TAF10, Myc, and BRCA1. In addition, it has been reported that ANLN is present in the nucleus to regulate the cell’s movement and infiltration by regulating the signal of the cytoplasm [114]. FLI1, friend leukemia integration 1 transcription factor; SWI/SNF, Switch/Sucrose non-fermentable; FLNA, Filamin A; BRCA1, breast cancer type 1; BRCA2, breast cancer type 2, ANLN, anillin actin-binding protein; Myc, a family of regulator genes and proto-oncogenes that code for transcription factors; TAF10, TATA-Box-binding protein associated factor 10; ACTN4, α-actinin 4; ERα, Estrogen receptor alpha; TF, transcription factor; Actin, a family of globular multi-functional proteins that form microfilaments.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12061600
This entry is offline, you can click here to edit this entry!
