Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Osteoporosis has been defined as the silent disease of the 21st century, becoming a public health risk due to its severity, chronicity and progression and affecting mainly postmenopausal women and older adults. Osteoporosis is characterized by an imbalance between bone resorption and bone production. It is diagnosed through different methods such as bone densitometry and dual X-rays. The treatment of this pathology focuses on different aspects.
- osteoporosis
- regenerative medicine
- lifestyle habits
1. Introduction
In 1993, the WHO defined osteoporosis as a systemic skeletal disease characterized by low bone mass, the deterioration of the microarchitecture of bone tissue, a consequent increase in bone fragility and a susceptibility to fractures [1]. In addition, osteoporosis has been reported to occur when there is an imbalance in bone cell function [2]. This disease has been called “the silent epidemic of the 21st century” because of its public health implications. It is a severe, chronic, progressive and clinically silent disease and the most common of the metabolic bone diseases [3].
Within osteoporosis, there are several types that can be classified into two large groups: primary and secondary osteoporosis. Primary osteoporosis includes idiopathic osteoporosis occurring in children and young adults, with an unknown etiopathogenesis [4], and involutional osteoporosis affects both men and women and is more related to aging [5]. Likewise, involutional osteoporosis is divided into type I or postmenopausal osteoporosis, which mainly affects women between 51 and 75 years of age and is characterized by rapid bone loss [6]. Type II or senile osteoporosis occurs in persons over 75 years of age and is characterized by a loss of trabecular and cortical bone that results from aging [3]. Secondary osteoporosis accounts for less than 5% of all cases of osteoporosis and is a consequence of a disease or the use of medications [7]. Among all of them, the most frequent kind of osteoporosis is postmenopausal osteoporosis, which is linked to two conditions: menopause and aging [6].
Among the metabolic bone diseases known to date, osteoporosis is not only the most frequent but is also a major global public health problem due to its high morbidity, which is caused by osteoporotic fractures in the older population [8]. This process occurs in people of both sexes and in the different types of osteoporosis, and it is also known to affect both pediatric and young patients, either primary or secondary to systemic diseases or medical treatments [9]. The National Institute Health Consensus on Prevention, Diagnosis and Therapy of Osteoporosis concluded that “bone mass acquired early in life may be the major determinant of long-term bone health” [10].
Due to the fact that bone loss is produced by advancing age, the prevalence of osteoporosis increases with it; therefore, as a chronic and prolonged skeletal disorder, it is more common in senile people, occurring in men over 65 years of age and in women over 55 years of age, approximately [11]. However, in women, it is more frequent due to other symptoms produced by menopause. During this stage, the estrogen deficit produces an increase in bone remodeling, which causes the loss of bone density [12]. In fact, in 2010, it was observed that 5.5 million men and 22 million women in the European Union had osteoporosis according to the diagnostic criteria used by the WHO [13], with 80% of the female population being unaware of the risk factors before being diagnosed with the disease [14].
Osteoporosis does not follow pre-established clinical patterns and manifests itself in various ways during its course. Individuals with uncomplicated osteoporosis may remain asymptomatic until a fracture occurs [5]. Although osteoporosis presents a general symptomatology, it also manifests with specific signs and symptoms such as: (i) pain that is secondary to osteoporotic fractures, which can occur in any bone and whose clinical manifestations depend on the location [15]; (ii) deformities and multiple vertebral compression fractures which can produce an increase in thoracic kyphosis and cervical lordosis [16]. The last ribs could contact the iliac crest, causing the relaxation of the diaphragm, which is the cause of digestive (hiatus hernia, meteorism) and respiratory (dyspnea) manifestations [17]. Moreover, there are alterations of the adipose panniculus and the presence of skin folds on the back, pubic region and umbilicus [18]. Likewise, hyperkyphosis causes cervical pain as the patient tries to keep the head upright through cervical hyperextension [19]. Moreover, increased dorsal kyphosis also occurs in osteoporotic males, resulting in shoulder droop, compensatory lumbar, cervical hyperlordosis and a characteristic postural habitus [20]. (iii) A loss of height, as vertebral fractures and hyperkyphosis can result in a decrease in height of about 10–20 cm, approximately [21].
Many factors are involved in the development of osteoporosis. Some of them are modifiable, such as environmental factors and some endocrine factors. Environmental factors include: (a) nutritional factors, such as deficient calcium intake, vitamin D deficiency due to nutritional problems, poor absorption or low sun exposure, excessive protein intake in unbalanced diets, excessive phosphate intake or excessive salt intake that increases urinary calcium loss [22]; (b) sedentary lifestyles, anaerobic exercise and excessive mechanical load, which are three factors that directly cause the risk of osteoporosis [23]; (c) chronic pharmacological treatment such as anti-convulsants, glucocorticoids, sedatives or chemotherapy; (d) the intake of caffeine, alcohol or smoking [24]; (e) body weight, which is responsible for 15% to 30% of the variations in bone mineral density (BMD) at any age and in any measured bone region [25]. Endocrine factors include: (a) late menarche or menstrual cycle alterations, which are conditions that are associated with low bone mass [26]; (b) surgical or non-surgical menopause before the age of 45 years [27]; (c) being a hormonally infertile woman [28]; and (d) estrogen deficiency before menopause as a result of anovulation due to anorexia nervosa, excessive exercise, mental stress, etc. This is the most important risk factor for osteoporosis, at least in Western countries [29]. It is important to look at these modifiable factors because they could be corrected and decrease the risk of developing osteoporosis [30].
In addition, there are non-modifiable risk factors such as genetics, since there are important genetic components in the determination of bone density and mass [31], e.g., race, since Caucasians and Asians are at a greater risk than Blacks and Polynesians [32]; sex, since it has been found that the risk is greater in women than in men [33]; and age, since each decade increases the risk by 1.4 to 1.8 times. It is another clear cause of bone density loss, not only because of the drop in hormone levels but also because, histologically, there is a decrease in the average thickness of the bone wall, but bone resorption remains high with aging [34].
2. Bone Biology
Bone tissue is a dynamic, mineralized connective tissue that serves multiple physiological functions [36]. Bone provides mechanical support for loading and locomotion, offers physical protection to internal soft organs, forms a non-static reservoir of calcium and phosphate ions and provides an environmental niche for bone marrow and hematopoietic cell development.
In bone, there is a hierarchical structure with two separate phases: the organic matrix and the inorganic matrix [37]. The organic matrix is composed mainly of type I collagen, the fibers of which are linked by triple helix cross-links. It is this structure that provides the bone with resistance to longitudinal tensile forces as well as elasticity. On the other hand, the inorganic matrix is mineralized with hydroxyapatite and calcium phosphate crystals, which are located in the free voids of the organic matrix. This matrix is responsible for the stiffness of the bone and its resistance to compressive forces in a way that depends on the amount of mineral, the arrangement of the crystals and the degree of packing [38].
The remaining bone volume is composed of bone cells of two classes: osteoprogenitor cells and osteoclasts. Osteoprogenitor cells are derived from mesenchymal stem cells (MSCs) that subsequently differentiate into osteoblasts and osteocytes. The differentiation of these cells is initiated when they receive migration signals to a certain area, proliferate and, finally, differentiate. Osteoblasts are the cells that line the surfaces of bone and are responsible for the synthesis and secretion of the organic bone matrix. Osteocytes are the majority of bone cells capable of communicating directly with each other [39,40]. All these cells are responsible for maintaining the bone matrix and regulating calcium homeostasis, although they also play an important role in bone resorption. Finally, osteoclasts are the largest cells, have multiple nuclei and are of hematopoietic origin. They are bone resorption cells and act by phagocytosing the matrix through acidification solubilization [41,42].
Furthermore, bone tissue can be differentiated into cortical bone or trabecular bone. Both types of bone are similar in their cellular and molecular composition but different in terms of functionality and mechanical characteristics [43]. Cortical bone is the bone found in the outermost part of the long bones. It is a very compact tissue that circulates the blood vessels, the canaliculi that surround the osteocytes and their connecting cellular processes. On the contrary, trabecular bone, also called cancellous bone, is found in the epiphysis of long bones, in the vertebrae and near the articular surfaces. It consists of a network of thin bony plates and connecting struts surrounded by the bone marrow [44].
Bone remodeling begins in fetal life and continues throughout our lives, adapting the shape of bones by removing and adding bone tissue at different key points [45]. Bone remodeling is crucial for the repair of bone damaged by constant physical loading and the prevention of fractures of various origins. This process is based on the balance of two main phases: bone formation and bone resorption (Figure 1) [46]. Bone is unique in the healing of connective tissue because it is capable of complete healing through cell regeneration and mineral matrix production [47]. As mentioned above, bone tissue is in a constant process of remodeling, which allows the skeleton to renew itself continuously. This remodeling process is directly related to mechanical stresses. This can prevent excessive fatigue damage, ensure the viability of bone cells, repair microfractures or allow for proper calcium homeostasis. The constant changes in bone mass and architecture due to load-bearing are regulated by osteoclasts together with the osteoblast–osteocyte communication system [48]. These bone cells form the main mechanical sensor network of the tissue.
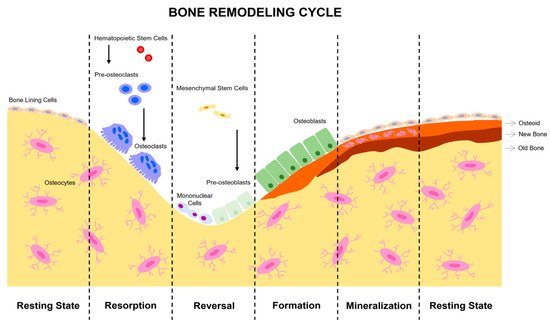
Figure 1. Stages of bone remodeling. In a balanced system, bone remodeling begins with bone resorption and ends with osteoblast formation. The complete cycle is composed of the phases of activation, resorption, reversion, formation and, finally, mineralization. Initially, a signal is detected which activates resorption by attracting osteoclast precursors to the area to be remodeled. This phase is of limited duration and depends on the degree of stimuli received, causing osteoclast differentiation and activity. Then, in the reversion phase, almost all of the osteoclasts disappear, and osteoblast precursors of mesenchymal origin begin to form. In the formation phase, all the osteoclasts are definitively replaced by osteoblasts. Finally, the mineralization of new bone tissue occurs. The new tissue remains at rest until the next cycle of remodeling.
During the remodeling process, there are several markers by which we can identify the existence of bone formation. Some of these markers are indicators of osteoblastic activity and the resulting metabolism after collagen release [49]. Alkaline phosphatase (ALP) is an enzyme associated with the plasma membrane of cells produced by osteoblasts, which play an important role in osteoid formation and mineralization. Its absence can lead to the development of liver disease. Osteopontin (OPN) is another non-collagenous protein with a key role in the structure and mechanics of bone tissue. A charged and phosphorylated protein with a high affinity for calcium, it has been attributed multiple functional roles in bone mineral bioregulation. It acts as a link at the mineral–collagen interface, improving bone hardness [50]. Another useful marker is osteocalcin (OC). OC is the most abundant non-collagenous protein present in the bone matrix. It is a small hydroxyapatite-binding protein synthesized by osteoblasts. It is generated during bone formation and can be released during bone resorption. This protein is rapidly degraded in vivo and ex vivo. Collagen type I is a protein synthesized by the osteoblasts and also serves as a bone formation marker [51].
Disuse can lead to deterioration in bone density and architecture, but physical exercise can slow the progression of these problems [52]. The mechanical forces supported by the cells in this tissue type are complex and multifactorial systems. The response of cells to these forces is regulated by cytoskeletal proteins and transmembrane-bound integrins that link the extracellular microenvironment with the genetic load in the nucleus. The bone marrow is also indirectly involved in bone remodeling. It produces MSCs that are also subjected to these loads, along with dynamic shear forces derived from the bone marrow bone interface. It is precisely these forces that promote osteogenesis and the cell differentiation of MSCs into an osteoblast lineage by dynamically activating the actin structure in the cytoskeleton while inhibiting adipogenesis [53].
Over the years, bones become more fragile and lose their functionality [54]. Factors such as immobilization, hormonal or nutritional deficiencies or chronic diseases can metabolically affect bone remodeling leading to osteopenia [55]. Therefore, the regulation of cellular and molecular processes to maintain the balance between bone resorption and bone formation is fundamental. An imbalance in this process can lead to the loss of bone density and mineral homeostasis, resulting in osteoporosis [56]. Osteoclasts, osteoblasts and osteocytes are bone cells directly involved in bone remodeling and a failure in their molecular mechanism is the possible trigger of the disease [57,58].
Several hormones, primarily estrogens [59], are responsible for regulating bone remodeling by controlling the cytokines and growth factors produced by bone marrow and bone cells. However, bone remodeling is also regulated by other systemic regulators (parathyroid hormone (PTH), vitamin D, calcitonin or glucocorticoids) and local regulators such as cytokines, growth factors mainly transforming growth factor beta (TGF-β), the macrophage colony-stimulating factor (M-CSF), receptor activators of nuclear factor-κB ligand (RANKL) or prostaglandins [58,60].
It is well known that the loss of estrogens increases bone resorption in women and, to some extent, in men. This is only supered by age-related osteoporosis. There are various factors, such as the low absorption of calcium and vitamin D and aging, which cause a decrease in the production of estrogens. The main cause of osteoporosis in women is menopause due to sex hormones reduction. A low production of estrogens causes the prolonged maintenance of osteoclasts. while osteoblastic cells deteriorate, leading to a homeostatic imbalance of the bone [61].
The action of these systemic regulators such as vitamin D and calcium exchange is essential for the physical resistance of bone and is closely related to PTH, one of most prominent regulatory hormones. Vitamin D levels are inversely related to PTH; if existing vitamin D decreases, PTH increases. This leads to a negative calcium balance and, consequently, to the deterioration of bone tissue [62].
Functional PTH receptors are found in osteoblasts, regardless of their maturation state. Problems in PTH regulation, such as ongoing hyperparathyroidism, result in a severe loss of bone mass, even though bone formation by osteoblasts continues. Although it is known that PTH plays a fundamental role in bone remodeling, it is not possible to determine how it is able to promote bone formation, since there is no single mechanism that explains it but rather multiple complementary mechanisms that act in a coordinated manner [63]. PTH anabolic treatments were the first Food and Drug Administration (FDA)-approved osteoporosis medications that could stimulate new bone formation [64].
On the other hand, the main signaling pathways controlling osteoclastic bone resorption and osteoblastic bone formation are the receptor activators of nuclear factor-κB (RANK)/RANKL/osteoprotegerin (OPG) and the canonical Wnt signaling [65,66].
First, for the initiation of the RANKL/RANK/OPG signaling pathway to occur, there must be an adequate concentration of M-CSF, which is expressed by osteocytes and osteoblasts. It stimulates the expression of RANK necessary before the action of RANKL. Subsequently, the binding of RANKL to its receptor on osteoclast precursor cells drives osteoclast differentiation, facilitating their activation and survival. RANKL/RANK binding induces a cascade of protein signaling molecules to enable osteoclast gene expression. RANKL produced by osteocytes is thought to sense changes in tissue load and initiate the bone remodeling cycle by stimulating osteoclastogenesis. Finally, OPG is a RANK receptor secreted by osteoblasts and osteocytes capable of inhibiting osteoclastic bone resorption by binding to RANKL instead of RANK [56]. The other key signaling pathway, the canonical Wnt pathway, is dependent on β-catenin, an important regulator of osteoblastic bone formation [65]. In the absence of Wnt, the cytoplasmic β-catenin glycoprotein is marked by proteasomal degradation that phosphorylates and ubiquitinates β-catenin. Because of this, the expression of the Wnt target gene is inhibited. If Wnt is present, it binds to a dual receptor complex comprising the Frizzled family of proteins, a seven transmembrane domain receptor and a lipoprotein-related co-receptor (LPL) (5 or 6). This blocks the action of the destruction complex, leading to the accumulation of cytoplasmic β-catenin, which ultimately promotes osteoblast proliferation and differentiation [56].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169465
This entry is offline, you can click here to edit this entry!
