Microalgae are photosynthetic organisms known for producing valuable metabolites under different conditions such as extreme temperatures, high salinity, osmotic pressure, and ultraviolet radiation. Microalgae can produce organic metabolites, such as sporopollenin, scytonemin, and mycosporine-like amino acids (MAAs) to mention a few, and these metabolites have the purpose to protect the microalgae from ultraviolet (UV) radiation while allowing visible radiation involved in photosynthesis to pass through. This characteristic allows some microalgae to be tolerant to UV rays. The understanding of these secondary metabolites produced by algae has led to the creation of promising industrially relevant compounds. The growing concern regarding the ecologically and dermatologically noxious implications of current sun protectants has brought with it the need for a safer alternative. MAAs are the most studied photo-protectant in marine organisms due to their relevance. They are abundant in various aquatic and terrestrial environments and are known for being eco-sustainable compounds since they are the outcome of the evolution of algae. Unlike their synthetic counterparts used unconventional sunscreens, there are no possibilities for pollution caused by them. Thus, algal metabolites shape the safety and sustainability profiles of commercial sunscreens.
- microalgae
- photo protectants
1. Introduction
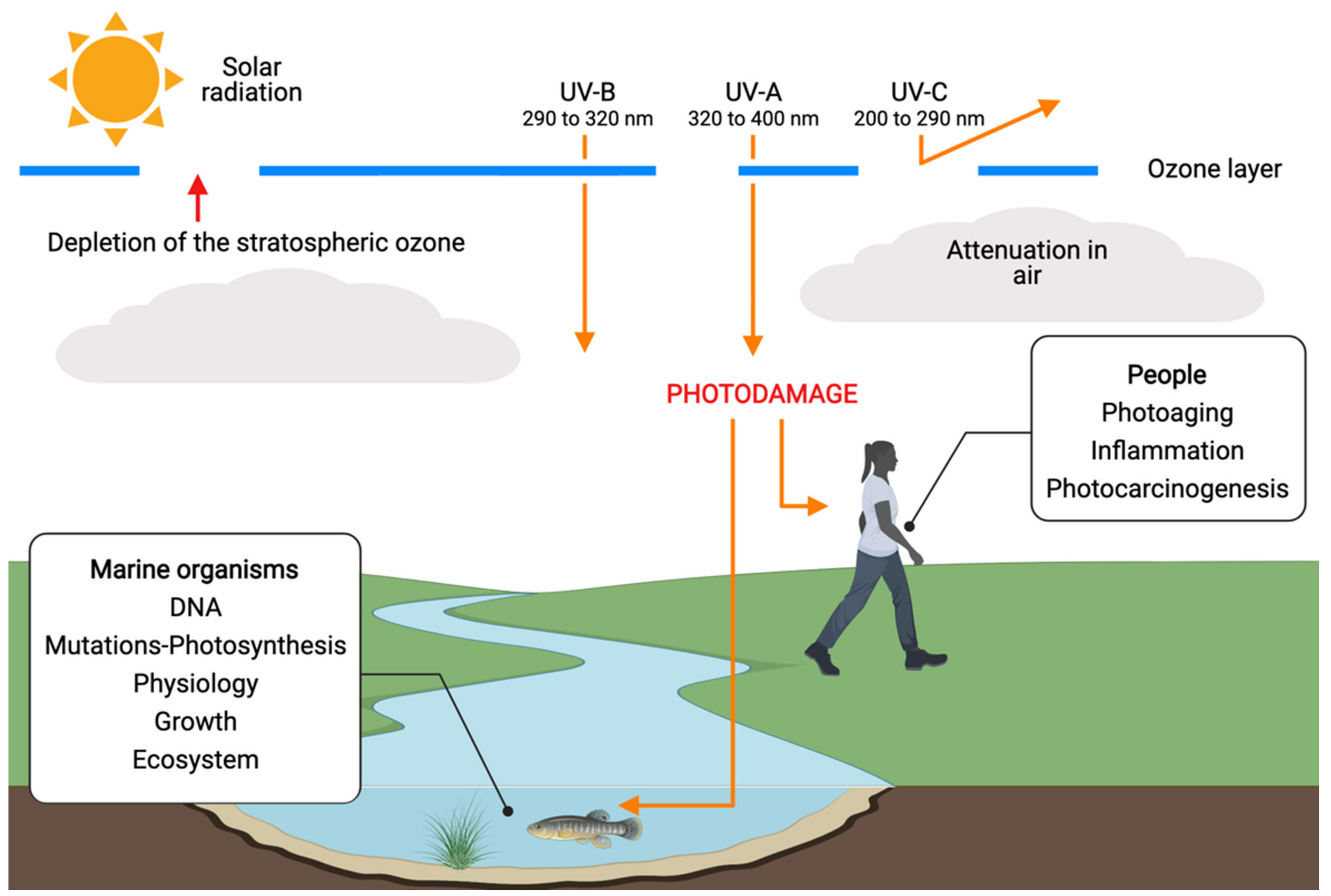
2. UV-Resistant Microalgae
3. UV-Induced Microalgae Biosynthesis
3.1. Mycosporine-Like Amino Acids (MAAs)
3.2. Carotenoids
3.3. Sporopollenin
3.4. Scytonemin
3.5. Phenolic Compounds
4. Bio-Carriers for Skin Applications
| Material Used | Microalgae | Component Loaded | Formulation | Purpose | Types of Tests | References |
|---|---|---|---|---|---|---|
| Chitosan | Spirulina | 5-Fluorouracil | Chitosan (1%(v/v)), Spirulina microalgae and genipin. | Drug delivery | In vitro cytotoxicity test | [39] |
| Alginate and Chitosan | Auxonochlorella protothecoides |
Microalgae oil extract | PVA solution (7–8% (w/v)) with sodium alginate (2% (w/v)) and PVA solution (7–8% (w/v)) with chitosan (2–3% (w/v)) | Nanoparticle production to deliver bioactive compounds in microalgae | In vitro release testing | [40][41][42] |
| Alginate, PVA | Botryococcus braunii and Microcystis aeruginosa |
Microalgae oil extract | PVA solution (8% (w/v)) and sodium alginate (2% (w/v)) | Nanoparticle production to deliver bioactive compounds in microalgae | In vitro release profile of nanoparticles | [40][43] |
| Alginate, cellulose | Chlorella vulgaris | Tea tree essential oil | Microfibrillated cellulose, nanofibrillated cellulose and carboxymethylcellulose (0.01% (m/v)) with alginate (2% (m/v)) | Cosmetic application | Differential scanning calorimetry | [44] |
| Cellulose | Chlorella pyrenoidosa | - | Microalgae powder, sodium dodecyl sulfate | Sensor for pollutant detection | - | [45] |
| Gelatin | Phaeodactylum tricornutum | Microalgae extract | P. tricornutum powder (0.5% or 1.0% (w/v)), gelatin solution | Wound dressing with antimicrobial P. tricornutum-loaded gelatin nanofiber mat | In vitro studies for antibacterial activity | [46][47] |
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/md20080487
References
- Roy, S. Impact of UV Radiation on Genome Stability and Human Health. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 996.
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187.
- Totonchy, M.B.; Chiu, M.W. UV-based therapy. Dermatol. Clin. 2014, 32, 399–413.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986.
- De Gruijl, F.R.; van Kranen, H.J.; Mullenders, L.H. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 19–27.
- Poon, T.S.C.; Barnetson, R.S.; Halliday, G.M. Prevention of Immunosuppression by Sunscreens in Humans Is Unrelated to Protection from Erythema and Dependent on Protection from Ultraviolet A in the Face of Constant Ultraviolet B Protection. J. Investig. Dermatol. 2003, 121, 184–190.
- Halliday, G.M.; Agar, N.S.; Barnetson, R.S.C.; Ananthaswamy, H.N.; Jones, A.M. UV-A Fingerprint Mutations in Human Skin Cancer. Photochem. Photobiol. 2005, 81, 3–8.
- Fourtanier, A.; Gueniche, A.; Compan, D.; Walker, S.L.; Young, A.R. Improved Protection Against Solar-Simulated Radiation-Induced Immunosuppression by a Sunscreen with Enhanced Ultraviolet A Protection. J. Investig. Dermatol. 2000, 114, 620–627.
- Radrezza, S.; Carini, M.; Baron, G.; Aldini, G.; Negre-Salvayre, A.; D’Amato, A. Study of Carnosine’s effect on nude mice skin to prevent UV-A damage. Free Radic. Biol. Med. 2021, 173, 97–103.
- Dutra, E.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. De Cienc. Farm. 2004, 40, 381–385.
- Alonso, C.; Barba, C.; Rubio, L.; Scott, S.; Kilimnik, A.; Coderch, L.; Notario, J.; Parra, J.L. An ex vivo methodology to assess the lipid peroxidation in stratum corneum. J. Photochem. Photobiol. B Biol. 2009, 972, 71–76.
- Sauce, R.; Pinto, C.A.S.D.O.; Ayala-Jara, C.; Prieto, Z.A.; Velasco, M.V.R.; Baby, A.R. Preliminary protocol development of a hplc-tbars-evsc (Ex vivo stratum corneum) assay for skin research: Application in a sunscreen system. Sci. Pharm. 2021, 89, 17.
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112.
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and self-regulation processes of microalgae under UV radiation stress. J. Photochem. Photobiol. C Photochem. Rev. 2019, 43, 100322.
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the southern ocean. Mar. Drugs 2018, 16, 336.
- Buma, A.G.; Wright, S.W.; van den Enden, R.; van de Poll, W.H.; Davidson, A.T. PAR acclimation and UVBR-induced DNA damage in Antarctic marine microalgae. Mar. Ecol. Prog. Ser. 2006, 315, 33–42.
- Xiong, F.; Kopecky, J.; Nedbal, L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta). Aquat. Bot. 1999, 63, 37–49.
- Kováčik, J.; Klejdus, B.; Bačkor, M. Physiological responses of Scenedesmus quadricauda (Chlorophyceae) to UV-A and UV-C light. Photochem. Photobiol. 2010, 86, 612–616.
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638.
- Fouqueray, M.; Mouget, J.L.; Morant-Manceau, A.; Tremblin, G. Dynamics of short-term acclimation to UV radiation in marine diatoms. J. Photochem. Photobiol. B Biol. 2007, 89, 1–8.
- Hernando, M.; Schloss, I.; Roy, S.; Ferreyra, G. Photoacclimation to long-term ultraviolet radiation exposure of natural sub-antarctic phytoplankton communities: Fixed surface incubations versus mixed mesocosms. Photochem. Photobiol. 2006, 82, 923–935.
- Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90.
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17.
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Long, P.F. O-Methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 2015, 16, 320–327.
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.; Carrillo-Nieves, D. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684.
- Pathak, J.; Ahmed, H.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172.
- Conde, F.; Churio, M.; Previtali, C. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144.
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring mycosporine-like amino acids (MAAs) as safe and natural protective agents against UV-induced skin damage. Antioxidants 2021, 10, 683.
- Zaki, N.A.A.; Mahmud, S.; Omar, A.F. Ultraviolet protection properties of commercial sunscreens and sunscreens containing zno nanorods. J. Phys. Conf. Ser. 2018, 1083, 012012.
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants 2021, 10, 2601.
- Huang, J.J.; Cheung, P.C. Enhancement of polyunsaturated fatty acids and total carotenoid production in microalgae by ultraviolet band A (UVA, 365 nm) radiation. J. Agric. Food Chem. 2011, 59, 4629–4636.
- Dionisio-Sese, M.L. Aquatic microalgae as potential sources of UV-screening compounds. Philipp. J. Sci. 2010, 139, 5–16.
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327.
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913.
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Cryst. Solids 2018, 481, 486–493.
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47.
- Huang, H.J.; Yuan, W.K.; Chen, X.D. Microencapsulation based on emulsification for producing pharmaceutical products: A literature review. Dev. Chem. Eng. Miner. Process. 2006, 14, 515–544.
- Khanmohammadi, M.; Elmizadeh, H.; Ghasemi, K. Investigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran. J. Pharm. Res. IJPR 2015, 14, 665.
- Aydınoğlu, D.; Ünal, M. Evaluation of the influence of spirulina microalgae on the drug delivery characteristics of genipin cross-linked chitosan hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1020–1033.
- Gao, Y.; Zhao, D.; Chang, M.W.; Ahmad, Z.; Li, J.S. Optimising the shell thicknessto-radius ratio for the fabrication of oil-encapsulated polymeric microspheres. Chem. Eng. J. 2016, 284, 963–971.
- Karakaş, C.Y.; Özçimen, D. A novel approach to production of Chlorella protothecoides oil loaded nanoparticles via electrospraying method: Modelling of critical parameters for particle sizing. Biotechnol. Appl. Biochem. 2020, 68, 659–668.
- Agrawal, P.; Pramanik, K. Chitosan-poly(vinyl alcohol) nanofibers by free surface electrospinning for tissue engineering applications. Tissue Eng. Regen. Med. 2016, 13, 485–497.
- İnan, B.; Özçimen, D. Preparation and characterization of microalgal oil loaded alginate/poly (vinyl alcohol) electrosprayed nanoparticles. Food Bioprod. Process. 2021, 129, 105–114.
- Morais, F.P.; Simões, R.M.S.; Curto, J.M.R. Biopolymeric Delivery Systems for Cosmetic Applications Using Chlorella vulgaris Algae and Tea Tree Essential Oil. Polymers 2020, 12, 2689.
- Bi, L.; Chen, Y.P.; Wang, C.; Su, J.; Pan, G. Microalgae-derived cellulose/inorganic nanocomposite rattle-type microspheres as an advanced sensor for pollutant detection. Chem. Eng. J. 2020, 395, 125073.
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917.
- Kwak, H.W.; Kang, M.J.; Bae, J.H.; Hur, S.B.; Kim, I.S.; Park, Y.H.; Lee, K.H. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int. J. Biol. Macromol. 2014, 63, 198–204.
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63.
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638.
- Yousuf, A. Fundamentals of Microalgae Cultivation. In Microalgae Cultivation for Biofuels Production; Academic Press: New York, NY, USA, 2020; pp. 1–9.
