The food industry is quite interested in the use of (techno)-functional bioactive compounds from by-products to develop ‘Clean label’ foods in a circular economy. Most studies are mainly focused on ultrasound extraction, which has been widely developed compared to microwave or enzymatic extractions, which should be deeply studied including combinations. After extraction, pomegranate peel by-products (in powders, liquid extract, and/or encapsulated, among others) have been incorporated into several food matrixes, as a good tool to preserve ‘Clean label’ foods without altering their composition and improving their functional properties. Future studies must clearly evaluate the energy efficiency/consumption, the cost, and the environmental impact leading to sustainable extraction of the key bio-compounds. Moreover, predictive models are needed to optimize the phytochemical extraction to help taking decisions along the supply chain
- Punica granatum
- peel
- food
- circular economy
- sustainability
- antioxidants
- phenolics
- encapsulation
- green-technology
- food losses
- clean label
- minimally processed
1. Introduction
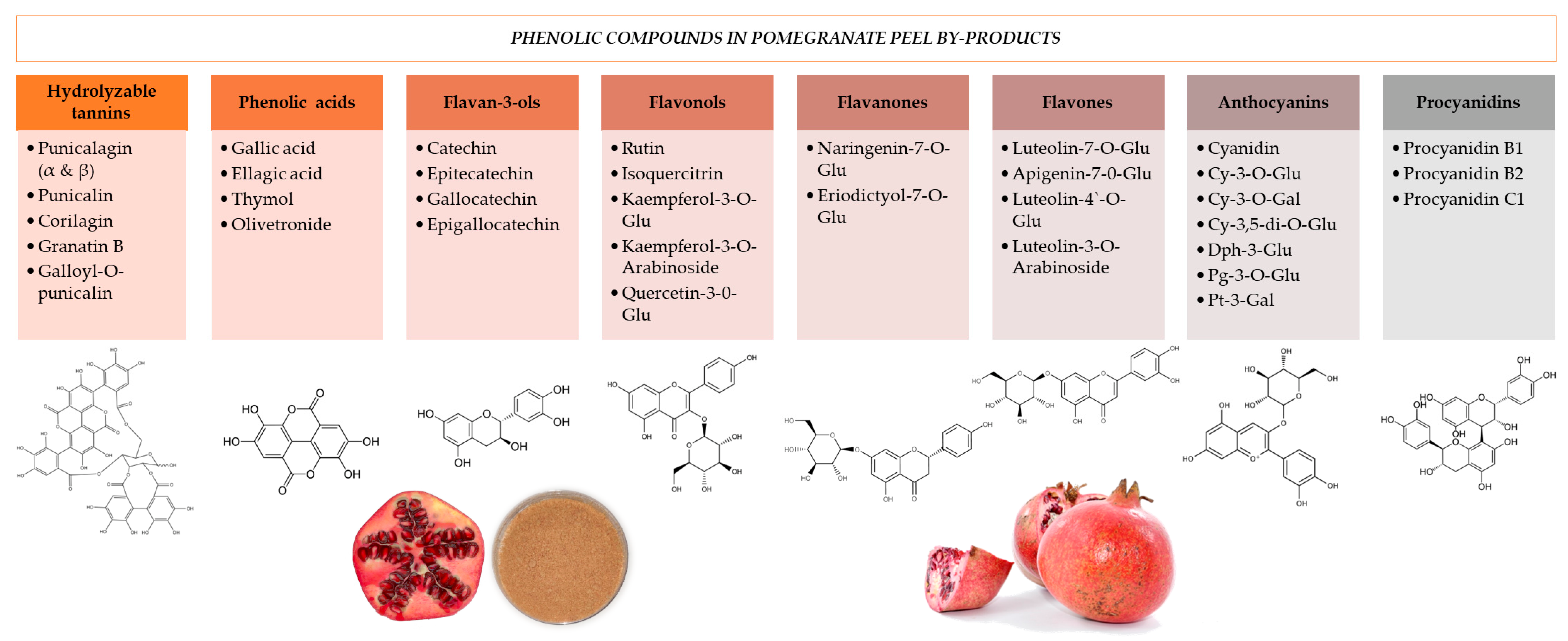
2. Nutritional Composition of Pomegranate Byproducts
3. Pomegranate Peel Byproducts Incorporation Techniques
3.1. Powders/Flours
3.2. Liquid Extracts
3.3. Encapsulation
| Technology | Carriers | Target Compound/Activity | Ref. |
|---|---|---|---|
| Spray-drying | Maltrodextrin | F-TPC, UPLC-TPC, Pn, EA, P, GA | [23][24] |
| Maltrodextrin + others: Tween 80 (99:1); Skimmed milk powder (50:50); Whey protein isolate (50:50); Gum arabic (50:50) | NA (Yield/Stability) | [25][26] | |
| Skimmed milk power | NA (Yield/Stability) | [25][26] | |
| Orange juice byproduct | F-TPC, DPPH | [27][28] | |
| Maltodextrin/Pectin | TPC, Pn, EA | [29] | |
| Whey protein | Pn, EA, P, GA | [24] | |
| Arabic gum | Pn, EA | [30] | |
| Chitosan | Pn, EA | [30][31] | |
| Pectin | Pn, EA | [30] | |
| Modified starch | Pn, TPC, HTC, DPPH | [32] | |
| Alginate | NA (Yield/Stability) | [31] | |
| Freeze-drying | Soy phosphatidylcholine liposomes | Pn, EA, rutin, epifallocatechin, TPC | [33] |
| Maltodextrin (5 and 10%) and b-cyclodextrin (5 and 10%). | F-TPC, FRAP | [34] | |
| Prunus armeniaca gum exudates | FRAP, DPPH | [35] | |
| Chitosan | FRAP, DPPH | [35] | |
| Maltrodextrin | TPC, TFC, Pn, EA, FRAP, DPPH | [36] | |
| Maltodextrin and calcium alginate | ANCs, FRAP, DPPH | [37] | |
| Maltodextrin and soy lecitin | NA (Yield/Stability) | [38] | |
| Double emulsion | Water1 in Oil in Water2: Water1 (ethanolic solutions) in Oil (castor, soybean, sunflower, medium chain triglyceride and orange) in Water2 (aqueous solution with Tween80) |
NA (Yield/Stability) | [39] |
| Ion gelation | Chitosan gel (1%):gelatin 2:1 | F-TPC, DPPH | [40] |
| Spirulina | TPC, DPPH | [17] | |
| Microalgae | EA | [41] | |
| Chitosan + others: Dialdehyde guar gum Gelatin-based materials |
F-TPC, DPPH | [42] |
4. Potential Applications in the Food Industry
4.1. Fresh Whole F&V
4.2. Minimally Processed, or Fresh-Cut F&V
4.3. F&V Based Beverages
| Matrix | Pomegranate Peel Byproduct |
Extraction | Incorporation Method | Benefit | Ref. | |
|---|---|---|---|---|---|---|
| Fresh whole F&V (pre- and postharvest) | Tomato | Drier (50–60 °C, 72 h) Fine powder (more information NA) cv information NA |
Ratio 3:10 EtOH 48 h + evaporator (65 °C) + re-dissolved in sterile distilled water (0.05%, 0.5%, 1% and 5% w/v) |
Preharvest. Tomato plants were sprayed in the leaves (bacteria inoculation) with the aqueous extract + 24 h drying |
Antibacterial activity at least 15 days Replacing, reducing, or even alternating treatments involving copper compounds |
[56] |
| Sage herb | Air dried (more information NA) Grinder (more information NA) cv information NA |
1:10 solid–liquid ratio in water or EtOH 80% 24 h + evaporator + water dilution | Preharvest. Added in the soil (2, 4, and 6 g per plot) |
Higher dry mass and essential oils Inhibition of free radical scavenging |
[57] | |
| Olive | Oven drier (40 °C) Powder home grinder (more information NA) Wonderful cv |
120 g/L EtOH solvent (50 and 80%) + 1% Citric acid | Postharvest. Treatment of 1 × 1-mm injuries and inoculated (C. acutatum) by 10 µL of pomegranate peel extract (12, 1.2, or 0.12 g/L) |
Reduction of fungal and bacterial population | [58] | |
| Potato tubers | Air drier (28 °C, 10–15 days) Fine powder (more information NA) Baladi cv |
1:10 solid–liquid (MetOH) 48 h 28 °C + evaporator + oven 50 °C 48 h | Postharvest. Wound (3 × 3 mm φ and deep) + inoculation (F. sambucinum) (24 h) + dipping (1.25, 2.5, 5, 10, and 20 mg/mL water) + air dried (2 h at 28 °C). |
Antifungal activity on the mycelial growth and spore germination | [59] | |
| Strawberry | Drying and particle size information NA Dente di caballo cv |
US 40 °C 80% A 3 min (3 on, 8 off) Ratio 1:10 (H2O 25%, propanol 25%, ethanol 25% and methanol 25%) + evaporator + Freeze-drier + re-dissolved in water |
Postharvest. Immersion (30 s in a 2 L solution of pomegranate peel extract) + air-drying (1 h) |
Extension of shelf life Substitution of synthetic pesticides |
[60] | |
| Sweet cherry | Oven drier (40 °C) Particle size NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% Citric acid +evaporator + Water dilution | Postharvest. Dipping (2 min) in the pomegranate extract (12, 2.4 or 1.2 g/L) + air drying (2 h, 28 °C) + storage at 1 °C |
Inhibition of all fungal spore germination | [61] | |
| Fresh whole F&V (pre- and postharvest) | Sweet cherry | Oven drier (40 °C) Fine powder < 470 µm cv information NA |
1:8 solid–liquid ratio (Water 28 °C 24 h) | Postharvest. Immersion in pomegranate peel extracts (3 min 20 °C) + room temperature drying |
Pomegranate peel extracts and calcium sulphate coatings, alone or in combination, decreased weight loss, decay, respiration rate, and increased acidity, firmness, ascorbic acid, DPPH, TPC, and TAC | [62] |
| Apple | Oven drier (40 °C) Particle size NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% citric acid + evaporator + water dilution | Postharvest. Wounds treated with 10 μL of pomegranate peel extract (12, 1.2 or 0.12 g/L) + inoculation (10 μL P. expansum) |
Inhibition of fungal spore germination and decay of artificial inoculations | [61] | |
| Mango | Freeze drying (−45 °C, 94 h) Particle size and cv information NA |
MetOH 45 °C 30 min + Bath US + evaporator + water dilution | Postharvest. Chitosan (2%) in 0.5% citric acid solution + Pullulan (2%) in water (50:50 ratios). During stiring: 1% glycerol + 5% of pomegranate peel extract (0.02 g/mL). Dipping for 2 min |
Increase of firmness, TPC and AOX. Prolonged the shelf life | [63] | |
| Apricot | Drier (60 °C, 48 h) Particle size < 0.251 mm cv information NA |
80% EtOH 25 °C + evaporator | Postharvest. Chitosan coating solution (1% chitosan in glacial acetic 1% + 0.8% glycerol + Tween 80 + 0.50, 0.75, and 1% pomegranate peel extract) |
Reduction of % decay and weight loss. Maintenance of DPPH radical scavenging activity, ascorbic acid content, titratable acidity and firmness. | [64] | |
| Figs | Air dried few days (more information NA) Pulverized (more information NA) cv information NA |
Alcoholic buffer (EtOH 50%) | Postharvest. Alginic acid: agar (70:30) + 0.25 and 0.5% pomegranate peel extract Dipping in the coating solution + coating gelation |
Prolonged the shelf life | [65] | |
| Dates | Drier (48 °C, 52 h) Ground peels (more information NA)cv information NA |
EtOH 70% + evaporator + Water dilution | Postharvest. 1% Chitosan, 1% nanochitosan or 1% pomegranate peel extract in 1% glacial acetic |
Growth inhibition of any fungal spore after 48 h of coating. | [66] | |
| Citrus | Hot air drier (50 °C, 48 h) Particle size 0.250 mm cv information NA |
2.5:10 Solid–liquid ratio (Ac, EtOH, MetOH, H2O, DMSO) + shaking (6 h) + re-extracted with water evaporation | Postharvest. Immersion of wounded lemons (2 × 1 mm long and wide tip) in pomegranate peel extract (pre-infection and post-infection with P. digitatum) + air drying |
Prevention and control of P. digitatum | [67] | |
| Fresh whole F&V (pre- and postharvest) | Grapefruit | Oven drier (40 °C) Particle size information NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% citric acid evaporator + water dilution |
Postharvest. Wounds treated with 10 μL of pomegranate peel extract (12, 1.2 or 0.12 g/L) + inoculation 10 μL P. digitatum and P. italicum |
Inhibition of all fungal spore germination and decay of artificial inoculations | [68] |
| Lemon | Oven drier (40 °C) Particle size information NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% citric acid + evaporator + water dilution | Postharvest. Wounds treated with 10 μL of pomegranate peel extract (12, 1.2 or 0.12 g/L) + inoculation 10 μL P. digitatum and P. italicum |
Inhibition of all fungal spore germination and decay of artificial inoculations | [61][68] | |
| Mandarin | Drier (70 °C, 48 h) Ground peels (more information NA) Shirine Shahvar cv |
0.25:10 solid–liquid ratio (60% EtOH + 0.1% citric acid) | Postharvest. Wounded (1 × 2 mm φ and depth) + dipping 1 min in pomegranate peel extract concentrations (25, 50, 75, 100%) + inoculation (P. italicum and P. digitatum) + drying |
Reduction of % infected wound and lesion φ (75% or/and 100% extract). Increase of TPC, TFC, and PAL activity (75% or/and 100% extract) | [69] | |
| Orange | Drier (35 °C, 2 days) Particle size NA Gabsi cv |
1:10, 0.6:10, 0.3:10 solid–liquid ratio (MetOH or Water) + evaporated + drying (40 °C or freeze-drying) + re-dissolved in water | Postharvest. Chitosan coating solution (1% chitosan in glacial acetic 1% + 0.5% Locust bean gum + 20% glycerol + 7, 18, and 36% dry waster/MetOH pomegranate peel extract). Wounded oranges (4 times: 3 × 3 mm φ × deep) + Inoculation (20 μL of a P. digitatum) + drying + dipping in different coating solutions (2 min) |
Controlled growth of Penicillium digitatum Reduction of postharvest decay |
[70] | |
| Orange | Oven drier (40 °C) Particle size NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% citric acid + evaporator + water dilution | Postharvest. Wounded oranges (3 times 2 × 2 mm φ and deep) + 20 μL pomegranate peel extract (12 g/L) + Inoculation (20 μL of a P. digitatum) + 1% citric acid + drying |
Enhanced defense pathways (antibiotic biosynthesis) | [71] | |
| Fresh whole F&V (pre- and postharvest) | Guava | Drier (60 °C, 72 h) Particle size 0.420 mm Bhagwa cv |
1:10 solid–liquid ratio (80% EtOH) + evaporation | Postharvest. Chitosan (1% chitosan in glacial acetic 1% + 0.75% glycerol) and alginate solution (2% alginate + 10% glycerol + 2% calcium chloride) with 1% pomegranate peel extract |
Preserved quality for 20 d under refrigeration | [72] |
| Capsicum | Drier (60 °C, 72 h) Particle size 0.420 mm Bhagwa cv |
1:10 solid–liquid ratio (80% EtOH) + evaporation | Postharvest. Chitosan (1% chitosan in glacial acetic 1% + 0.75% glycerol) and alginate solution (2% alginate + 10% glycerol + 2% calcium chloride) with 1% pomegranate peel extract |
Inhibition of microbial growth. Preserved sensory quality. Extension of shelf life up to 25 d at 10 °C |
[73] | |
| Pear | Drier (60 °C, 72 h) Particle size 0.420 mm Bhagwa cv |
1:10 solid–liquid ratio (80% EtOH) + evaporation | Postharvest. Chitosan (1% chitosan in glacial acetic 1% + 0.75% glycerol) and alginate solution (2% alginate + 10% glycerol + 2% calcium chloride) with 2% pomegranate peel extract |
Lowered the cell wall degrading enzymes activity (firmness preservation) | [74] | |
| Fresh-cut/Minimally processed F&V | Fruit salad: nectarine and pineapple in cubes covered with fructose syrup |
Oven drier (38 °C, 48 h) Particle size 500 mm Wonderful cv |
Powder | 2.5–5% (w/v) of pomegranate peel powder at the container bottom | Inhibition of mesophilic bacteria, total psychrotrophic microorganisms, yeasts, and lactic acid bacteria No negative effect on sensory characteristics |
[75] |
| Fresh-cut pear, apple and melon (plugs) |
Oven drier (40 °C) Particle size NA Mollar de Elche cv |
EtOH solvent (50 and 80%) + 1% citric acid + evaporator + water dilution | Inoculated plugs were dipped (10 min, 150 rpm) + dried (25 °C 30 min) | Reduction of Listeria monocytogenes | [76] | |
| Fresh-cut Golden apple wedges: thickness 30-mm and 30 g |
Drying and particle size information NA Dente di cavallo cv |
Pulsed UAE (10 min, <50 °C, 1:40, 26 kHz, 200 W, 40% A, 50% duty cycle) + encapsulation with pectin from citrus peel by spray drying | Enrichment with microencapsulates reconstituted in water 1:1 | Reduction of enzymatic browning. Color preservation | [77] | |
| Beverages | Carrot juice | Oven drier (40 °C) Grounded in a colloid mill (more information NA) cv information NA |
High pressure-assisted extraction | 5 mg pomegranate peel extract per mL of carrot juice | Improvement of microbiological safety and AOX during storage. Color preservation | [78][79] |
| Beverages | Apple juice | Oven drier (55 °C, 12 h) Particle size and cv information NA |
Maceration extraction (1:50, 80% EtOH 1 h shaking) | Different% of pomegranate peel extract (0–2%) | Enhancing sensory quality and AOX. Low toxicity with 1% of pomegranate peel extract | [80] |
| Kiwi juice | Information NA | Commercial pomegranate extract (PureBulk, Roseburg) | Extract incorporation (180 μg/mL kiwi juice) + US bath (40 kHz, 180 W, 20 °C, 10–30 min) | US and pomegranate extract combined treatment: higher reductions on yeast and molds | [81] | |
| Red wine | Green decoction: Boiled in water 60 min (1:40) Freeze-drying of the extract Wonderful cv |
Powder | Purification to obtain the tannins. 8 analyzed tannins (1 g L−1 wine solution) |
Increase of protein stability Increase of color stability Reduction of sulfites |
[82] | |
| Symbiotic drink powder |
Hot oven (40 °C, 48 h) Particle size Kitchen-miller (more information NA) cv information NA |
Ethanolic extract (80%; 1:15) + evaporator + Freeze-drier | Formulation: beetroot peel extract powder (3%), pomegranate peel extract powder (1%), grape pomace extract powder (1.5%), quince seed gum (0.5%), stevia (4%), mint (0.1%) and water (89.9%). Pasteurization: 72 °C, 90 s | Maintenance of L. casei viability of the recommended level of 10−7 CFU/g | [83] |
This entry is adapted from the peer-reviewed paper 10.3390/foods11172596
References
- FAO. Technical Platform on the Measurement and Reduction of Food Loss and Waste. 2022. Available online: https://www.fao.org/platform-food-loss-waste/en/ (accessed on 20 July 2022).
- López–Gómez, A.; Ros–Chumillas, M.; Buendía-Moreno, L.; Martínez–Hernández, G.B. Active Cardboard Packaging with Encapsulated Essential Oils for Enhancing the Shelf Life of Fruit and Vegetables. Front. Nutr. 2020, 7, 559978.
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883.
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59.
- Turker, S.; Polat, A.A.; Bindak, R. Seasonal Changes of Carbohydrates in Fruit Peels, Leaves and Shoots of Three Pomegranate (Punica granatum L.) Cultivars Grown in Upper Euphrates Basin. Sci. Hortic. 2022, 304, 111315.
- Abid, M.; Renard, C.M.G.C.; Watrelot, A.A.; Fendri, I.; Attia, H.; Ayadi, M.A. Yield and Composition of Pectin Extracted from Tunisian Pomegranate Peel. Int. J. Biol. Macromol. 2016, 93, 186–194.
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An Integrated Green Biorefinery Approach towards Simultaneous Recovery of Pectin and Polyphenols Coupled with Bioethanol Production from Waste Pomegranate Peels. Bioresour. Technol. 2018, 266, 322–334.
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J.A. Chemical, Physico-Chemical and Functional Properties of Pomegranate (Punica granatum L.) Bagasses Powder Co-Product. J. Food Eng. 2012, 110, 220–224.
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859.
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657.
- Man, G.; Xu, L.; Wang, Y.; Liao, X.; Xu, Z. Profiling Phenolic Composition in Pomegranate Peel From Nine Selected Cultivars Using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front. Nutr. 2022, 8.
- Cano-Lamadrid, M.; Lech, K.; Calín-Sánchez, Á.; Rosas-Burgos, E.C.; Figiel, A.; Wojdyło, A.; Wasilewska, M.; Carbonell-Barrachina, Á.A. Quality of Pomegranate Pomace as Affected by Drying Method. J. Food Sci. Technol. 2018, 55, 1074–1082.
- Alcaraz-Mármol, F.; Nuncio-Jáuregui, N.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez, J.J.; Hernández, F. Determination of Fatty Acid Composition in Arils of 20 Pomegranates Cultivars Grown in Spain. Sci. Hortic. 2015, 197, 712–718.
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261.
- Viuda-Martos, M.; Pérez-Álvarez, J.A.; Sendra, E.; Fernández-López, J. In vitro antioxidant properties of pomegranate (Punica granatum) peel powder extract obtained as coproduct in the juice extraction process. J. Food Process. Preserv. 2013, 37, 772–776.
- Bhandari, B. 1-Introduction to Food Powders. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Shaston, UK, 2013; pp. 1–25. ISBN 978-0-85709-513-8.
- Yağmur, N.; Şahin, S. Encapsulation of Ellagic Acid from Pomegranate Peels in Microalgae Optimized by Response Surface Methodology and an Investigation of Its Controlled Released under Simulated Gastrointestinal Studies. J. Food Sci. 2020, 85, 998–1006.
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application–A Review. Trends Food Sci. Technol. 2021, 116, 11–23.
- Michalska, A.; Wojdyło, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-Induced Physico-Chemical Changes in Cranberry Products. Food Chem. 2018, 240, 448–455.
- Michalska, A.; Lech, K. The Effect of Carrier Quantity and Drying Method on the Physical Properties of Apple Juice Powders. Beverages 2018, 4, 2.
- Watson, M.A.; Lea, J.M.; Bett-Garber, K.L. Spray Drying of Pomegranate Juice Using Maltodextrin/Cyclodextrin Blends as the Wall Material. Food Sci. Nutr. 2017, 5, 820–826.
- Carpena, M.; Garcia-Oliveira, P.; Lourenco-Lopez, C.; Gonzalez Pereira, A.; Fraga-Corral, M.; Angel Prieto, M.; Simal-Gandara, J. Freeze-Drying Encapsulation as a Mechanism of Choice in Oils: Methods and Mechanism. In Basic Protocols in Encapsulation of Food Ingredients; Gomez-Zavaglia, A., Ed.; Springer: New York, NY, USA, 2021; pp. 91–101. ISBN 978-1-0716-1649-9.
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT-Food Sci. Technol. 2014, 55, 117–123.
- Šavikin, K.; Nastić, N.; Janković, T.; Bigović, D.; Miličević, B.; Vidović, S.; Menković, N.; Vladić, J. Effect of Type and Concentration of Carrier Material on the Encapsulation of Pomegranate Peel Using Spray Drying Method. Foods 2021, 10, 1968.
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A Process for Turning Pomegranate Peels into a Valuable Food Ingredient Using Ultrasound-Assisted Extraction and Encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215.
- Goula, A.M.; Lazarides, H.N. Integrated Processes Can Turn Industrial Food Waste into Valuable Food By-Products and/or Ingredients: The Cases of Olive Mill and Pomegranate Wastes. J. Food Eng. 2015, 167, 45–50.
- Kaderides, K.; Goula, A.M. Encapsulation of Pomegranate Peel Extract with a New Carrier Material from Orange Juice By-Products. J. Food Eng. 2019, 253, 1–13.
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of Pomegranate Peel Polyphenols Encapsulated in Orange Juice Industry By-Product and Their Incorporation in Cookies. Food Chem. 2020, 310, 125849.
- Yang, B.; Kealey, K.; Chen, J.; Solval, K.M. Developing Microencapsulated Powders Containing Polyphenols and Pectin Extracted from Georgia-Grown Pomegranate Peels. LWT 2022, 154, 112644.
- Fathi, F.; Ebrahimi, S.N.; Pereira, D.M.; Estevinho, B.N.; Rocha, F. Preliminary Studies of Microencapsulation and Anticancer Activity of Polyphenols Extract from Punica granatum Peels. Can. J. Chem. Eng. 2021.
- Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Activity of Spray-Dried Microparticles Containing Pomegranate Peel Extract against Candida Albicans. Molecules 2012, 17, 10094–10107.
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and Microencapsulation of Bioactive Compounds from Pomegranate (Punica granatum Var. Wonderful) Residues. Int. J. Food Sci. Technol. 2017, 52, 1452–1462.
- Marín, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Encapsulation of Food Waste Compounds in Soy Phosphatidylcholine Liposomes: Effect of Freeze-Drying, Storage Stability and Functional Aptitude. J. Food Eng. 2018, 223, 132–143.
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel. LWT 2019, 101, 342–350.
- Jafarian, M.; Taghinia, P.; Baeghbali, S.; Samadi Ghorbani Dozdozani, N. Application of Prunus Armeniaca Gum Exudates and Chitosan for Encapsulation of Pomegranate Peel Extract as a Natural Antioxidant. J. Food Meas. Charact. 2021, 15, 4992–4999.
- Hamid; Thakur, N.S.; Thakur, A. Microencapsulation of Wild Pomegranate Flavedo Phenolics by Lyophilization: Effect of Maltodextrin Concentration, Structural Morphology, Functional Properties, Elemental Composition and Ingredient for Development of Functional Beverage. LWT 2020, 133, 110077.
- Azarpazhooh, E.; Sharayei, P.; Zomorodi, S.; Ramaswamy, H.S. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-Dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol. 2019, 12, 199–210.
- Okumuş, E.; Bakkalbaşı, E.; Javidipour, I.; Meral, R.; Ceylan, Z. A Novel Coating Material: Ellagitannins-Loaded Maltodextrin and Lecithin-Based Nanomaterials. Food Biosci. 2021, 42, 101158.
- Sanhueza, L.; García, P.; Giménez, B.; Benito, J.M.; Matos, M.; Gutiérrez, G. Encapsulation of Pomegranate Peel Extract (Punica granatum L.) by Double Emulsions: Effect of the Encapsulation Method and Oil Phase. Foods 2022, 11, 310.
- Bertolo, M.R.V.; Martins, V.C.A.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M.G. Rheological and Antioxidant Properties of Chitosan/Gelatin-Based Materials Functionalized by Pomegranate Peel Extract. Carbohydr. Polym. 2020, 228, 115386.
- Yağmur, N.; Şahin, S.; Korkmaz, E. Microencapsulation of Ellagic Acid Extracted from Pomegranate Peel onto Spirulina: Characterization, Loading, and Storage Stability Properties. J. Food Process. Preserv. 2021, 45, e15086.
- Maroufi, L.Y.; Tabibiazar, M.; Ghorbani, M.; Jahanban-Esfahlan, A. Fabrication and Characterization of Novel Antibacterial Chitosan/Dialdehyde Guar Gum Hydrogels Containing Pomegranate Peel Extract for Active Food Packaging Application. Int. J. Biol. Macromol. 2021, 187, 179–188.
- Costa, A.M.M.; Moretti, L.K.; Simões, G.; Silva, K.A.; Calado, V.; Tonon, R.V.; Torres, A.G. Microencapsulation of Pomegranate (Punica granatum L.) Seed Oil by Complex Coacervation: Development of a Potential Functional Ingredient for Food Application. LWT 2020, 131, 109519.
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT 2018, 90, 254–264.
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of Pomegranate Peel Extract as a Natural Additive in Foods. Trends Food Sci. Technol. 2021, 115, 380–390.
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Designing a Clean Label Fish Patty with Olive, Citric, Pomegranate, or Rosemary Extracts. Plants 2020, 9, 659.
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86.
- Mekawi, E.M.; Sharoba, A.M.; Ramadan, M.F. Reduction of Acrylamide Formation in Potato Chips during Deep-Frying in Sunflower Oil Using Pomegranate Peel Nanoparticles Extract. J. Food Meas. Charact. 2019, 13, 3298–3306.
- Chan, C.-L.; Gan, R.; Shah, N.P.; Corke, H. Enhancing Antioxidant Capacity of Lactobacillus Acidophilus-Fermented Milk Fortified with Pomegranate Peel Extracts. Food Biosci. 2018, 26, 185–192.
- Palabiyik, I.; Toker, O.S.; Konar, N.; Gunes, R.; Güleri, T.; Alaşalvar, H.; Çam, M. Phenolics Release Kinetics in Sugared and Sugar-Free Chewing Gums: Microencapsulated Pomegranate Peel Extract Usage. Int. J. Food Sci. Technol. 2018, 53, 2657–2663.
- da Silva Veloso, F.; Caleja, C.; Calhelha, R.C.; Pires, T.C.S.; Alves, M.J.; Barros, L.; Genena, A.K.; Barreira, J.C.M.; Ferreira, I.C.F.R. Characterization and Application of Pomegranate Epicarp Extracts as Functional Ingredients in a Typical Brazilian Pastry Product. Molecules 2020, 25, 1481.
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Jekle, M.; Becker, T. Development of Fibre-Enriched Wheat Breads: Impact of Recovered Agroindustrial by-Products on Physicochemical Properties of Dough and Bread Characteristics. Eur. Food Res. Technol. 2017, 243, 1973–1988.
- Kumar, N.; Daniloski, D.; Pratibha; Neeraj; D’Cunha, N.M.; Naumovski, N.; Petkoska, A.T. Pomegranate Peel Extract–A Natural Bioactive Addition to Novel Active Edible Packaging. Food Res. Int. 2022, 156, 111378.
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L.; Martínez-Hernández, G.B. Phytochemical Fortification in Fruit and Vegetable Beverages with Green Technologies. Foods 2021, 10, 2534.
- Castillejo, N.; Martínez-Hernández, G.B.; Artés-Hernández, F. Revalorized Broccoli By-Products and Mustard Improved Quality during Shelf Life of a Kale Pesto Sauce. Food Sci. Technol. Int. 2021, 27, 734–745.
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological Control of Tomato Bacterial Speck Using Punica granatum Fruit Peel Extract. Crop Prot. 2013, 46, 18–22.
- Abd-Rabbu, H.S.; Wahba, H.E.; Khalid, K.A. Pomegranate Peel Modifies Growth, Essential Oil and Certain Chemicals of Sage (Salvia officinalis L.) Herb. Biocatal. Agric. Biotechnol. 2021, 33, 101978.
- Pangallo, S.; Nicosia, M.G.L.D.; Agosteo, G.E.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Evaluation of a Pomegranate Peel Extract as an Alternative Means to Control Olive Anthracnose. Phytopathology 2017, 107, 1462–1467.
- Elsherbiny, E.A.; Amin, B.H.; Baka, Z.A. Efficiency of Pomegranate (Punica granatum L.) Peels Extract as a High Potential Natural Tool towards Fusarium Dry Rot on Potato Tubers. Postharvest Biol. Technol. 2016, 111, 256–263.
- Rongai, D.; Sabatini, N.; Pulcini, P.; di Marco, C.; Storchi, L.; Marrone, A. Effect of Pomegranate Peel Extract on Shelf Life of Strawberries: Computational Chemistry Approaches to Assess Antifungal Mechanisms Involved. J. Food Sci. Technol. 2018, 55, 2702–2711.
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of Postharvest Fungal Rots on Citrus Fruit and Sweet Cherries Using a Pomegranate Peel Extract. Postharvest Biol. Technol. 2016, 114, 54–61.
- Parsa, Z.; Roozbehi, S.; Hosseinifarahi, M.; Radi, M.; Amiri, S. Integration of Pomegranate Peel Extract (PPE) with Calcium Sulphate (CaSO4): A Friendly Treatment for Extending Shelf-Life and Maintaining Postharvest Quality of Sweet Cherry Fruit. J. Food Process. Preserv. 2021, 45, e15089.
- Kumar, N.; Pratibha; Neeraj; Petkoska, A.T.; AL-Hilifi, S.A.; Fawole, O.A. Effect of Chitosan–Pullulan Composite Edible Coating Functionalized with Pomegranate Peel Extract on the Shelf Life of Mango (Mangifera indica). Coatings 2021, 11, 764.
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf Life Extension of Apricot Fruit by Application of Nanochitosan Emulsion Coatings Containing Pomegranate Peel Extract. Food Chem. 2021, 349, 129149.
- Paolucci, M.; di Stasio, M.; Sorrentino, A.; la Cara, F.; Volpe, M.G. Active Edible Polysaccharide-Based Coating for Preservation of Fresh Figs (Ficus carica L.). Foods 2020, 9, 1793.
- Alotaibi, M.A.; Tayel, A.A.; Zidan, N.S.; el Rabey, H.A. Bioactive Coatings from Nano-Biopolymers/Plant Extract Composites for Complete Protection from Mycotoxigenic Fungi in Dates. J. Sci. Food Agric. 2019, 99, 4338–4343.
- Tayel, A.A.; El-Baz, A.F.; Salem, M.F.; El-Hadary, M.H. Potential Applications of Pomegranate Peel Extract for the Control of Citrus Green Mould. J. Plant Dis. Prot. 2009, 116, 252–256.
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Rapisarda, P.; Droby, S.; Schena, L. Elicitation of Resistance Responses in Grapefruit and Lemon Fruits Treated with a Pomegranate Peel Extract. Plant Pathol. 2017, 66, 633–640.
- Givi, F.; Gholami, M.; Massah, A. Application of Pomegranate Peel Extract and Essential Oil as a Safe Botanical Preservative for the Control of Postharvest Decay Caused by Penicillium Italicum and Penicillium Digitatum on “Satsuma” Mandarin. J. Food Saf. 2019, 39, e12639.
- Kharchoufi, S.; Parafati, L.; Licciardello, F.; Muratore, G.; Hamdi, M.; Cirvilleri, G.; Restuccia, C. Edible Coatings Incorporating Pomegranate Peel Extract and Biocontrol Yeast to Reduce Penicillium Digitatum Postharvest Decay of Oranges. Food Microbiol. 2018, 74, 107–112.
- Belgacem, I.; Pangallo, S.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Li Destri Nicosia, M.G.; Ballistreri, G.; Schena, L. Transcriptomic Analysis of Orange Fruit Treated with Pomegranate Peel Extract (PGE). Plants 2019, 8, 101.
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of Chitosan and Alginate Based Coatings Enriched with Pomegranate Peel Extract to Extend the Postharvest Quality of Guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252.
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and Antifungal Activity of Pomegranate Peel Extract and Its Use in Polysaccharide-Based Edible Coatings to Extend the Shelf-Life of Capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327.
- Megha, M.; Gill, P.S.; Jawandha, S.K.; Kaur, N.; Gill, M.S. Effect of Chitosan Coating Incorporated with Pomegranate Peel Extract on Pear Fruit Softening, Quality, and Cell Wall Degrading Enzymes during Cold Storage. J. Food Process. Preserv. 2021, 45, e15984.
- Lacivita, V.; Incoronato, A.L.; Conte, A.; del Nobile, M.A. Pomegranate Peel Powder as a Food Preservative in Fruit Salad: A Sustainable Approach. Foods 2021, 10, 1359.
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.V.; Ballistreri, G.; Abadias, M. Effectiveness of a Pomegranate Peel Extract (PGE) in Reducing Listeria Monocytogenes in Vitro and on Fresh-Cut Pear, Apple and Melon. Eur. Food Res. Technol. 2020, 246, 1765–1772.
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From Pomegranate Marcs to a Potential Bioactive Ingredient: A Recycling Proposal for Pomegranate-Squeezed Marcs. Eur. Food Res. Technol. 2020, 246, 273–285.
- Trigo, J.P.; Alexandre, E.M.C.; Oliveira, A.; Saraiva, J.A.; Pintado, M. Fortification of Carrot Juice with a High-Pressure-Obtained Pomegranate Peel Extract: Chemical, Safety and Sensorial Aspects. Int. J. Food Sci. Technol. 2020, 55, 1599–1605.
- Trigo, J.P.; Alexandre, E.M.C.; Silva, S.; Costa, E.; Saraiva, J.A.; Pintado, M. Study of Viability of High Pressure Extract from Pomegranate Peel to Improve Carrot Juice Characteristics. Food Funct. 2020, 11, 3410–3419.
- Altunkaya, A.; Hedegaard, R.V.; Harholt, J.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Palatability and Chemical Safety of Apple Juice Fortified with Pomegranate Peel Extract. Food Funct. 2013, 4, 1468–1473.
- Tomadoni, B.; Moreira, M.D.R.; Espinosa, J.P.; Ponce, A. Individual and Combined Effects of Pomegranate Extract and Ultrasonic Treatments on Kiwifruit Juice Quality Parameters. J. Food Process Eng. 2017, 40, e12339.
- Canuti, V.; Cecchi, L.; Khatib, M.; Guerrini, L.; Mulinacci, N.; Zanoni, B. A New Extract from Pomegranate (Punica granatum L.) By-Products as a Potential Oenological Tannin: Preliminary Characterization and Comparison with Existing Commercial Products. Molecules 2020, 25, 4460.
- Jouki, M.; Khazaei, N.; Rashidi-Alavijeh, S.; Ahmadi, S. Encapsulation of Lactobacillus Casei in Quince Seed Gum-Alginate Beads to Produce a Functional Synbiotic Drink Powder by Agro-Industrial by-Products and Freeze-Drying. Food Hydrocoll. 2021, 120, 106895.
