Bone is a complex biologic tissue, which is extremely relevant for various physiological functions, in addition to movement, organ protection, and weight bearing. The repair of critical size bone defects is a still unmet clinical need, and over the past, material scientists have been expending efforts to find effective technological solutions, based on the use of scaffolds. In this context, biomimetics which is intended as the ability of a scaffold to reproduce compositional and structural features of the host tissues, is increasingly considered as a guide for this purpose. However, the achievement of implants that mimic the very complex bone composition, multi-scale structure, and mechanics is still an open challenge. Indeed, despite the fact that calcium phosphates are widely recognized as elective biomaterials to fabricate regenerative bone scaffolds, their processing into 3D devices with suitable cell-instructing features is still prevented by insurmountable drawbacks. With respect to biomaterials science, new approaches maybe conceived to gain ground and promise for a substantial leap forward in this field.
- biomimetics
- hydroxyapatite
- 3D scaffolds
- bone regeneration
1. Introduction
2. Translation of the Biomimetic Concept to 3D Scaffold Development
2.1. Limitations of Current Approaches and Further Challenges in Tissue Engineering
2.2. Guiding Bone Regeneration by Chemistry and Crystal Structure
2.2.1. Synthesis Processes for the Production of Biomimetic Apatites
2.2.2. Tailoring the Dissolution Mechanism and Solubility of Apatites
2.3. Guiding Bone Regeneration by 3D Scaffold Architecture and Porosity
3. Recent Approaches Yielding Biomimetic Ceramic-Based Scaffolds
3.1. Organic/Inorganic Scaffolds by 3D Printing
3.2. 3D Hybrid Scaffolds Using Natural Polymers and Bio-Inspired Mineralization Processes
3.3. Bioactive Glass Scaffolds
-
Leaching through the exchange of protons from the physiological medium with labile network-modifying ions, such as Na+, K+, Ca2+, Mg2+, etc.:
- 2.
-
The previous pH rise facilitates dissolution of the network and formation of additional silanol groups according to the reaction:
- 3.
-
Polymerization of the SiO2− rich layer through condensation of neighboring Si–OH groups, which produces a layer rich in amorphous silica.
- 4.
-
Migration of Ca2+ ions to the surface of the silica-rich layer to form an amorphous film rich in CaO–P2O5, followed by thickening of the film by incorporation of soluble Ca2+ and PO43− ions from the solution.
- 5.
3.4. Self-Hardening Apatitic Scaffolds
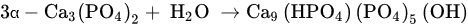
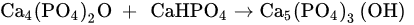
3.5. Mechanically Bearing, Biomorphic 3D Scaffolds
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics7030112
References
- Baroli, B. From Natural Bone Grafts to Tissue Engineering Therapeutics: Brainstorming on Pharmaceutical Formulative Requirements and Challenges. J. Pharm. Sci. 2009, 98, 1317–1375.
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone Allografts: What They Can Offer and What They Cannot. J. Bone Jt. Surg. Br. 2007, 89, 574–579.
- St John, T.A.; Vaccaro, A.R.; Sah, A.P.; Schaefer, M.; Berta, S.C.; Albert, T.; Hilibrand, A. Physical and Monetary Costs Associated with Autogenous Bone Graft Harvesting. Am. J. Orthop. 2003, 32, 18–23.
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac Crest Bone Graft Harvest Donor Site Morbidity. Spine. 1995, 20, 1055–1060.
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. In Tissue Engineering—Part B: Reviews; Mary Ann Liebert Inc.: Larchmont, NY, USA, 2019; pp. 375–386.
- Vivanco, J.; Araneda, A.; Ploeg, H.-L. Effect of Sintering Temperature on Microstructural Properties of Bioceramic Bone. Biomater. Sci. Process. Prop. Appl. II 2012, 237, 101–109.
- Pramanik, S.; Agarwal, A.K.; Rai, K.N.; Garg, A. Development of High Strength Hydroxyapatite by Solid-State-Sintering Process. Ceram. Int. 2007, 33, 419–426.
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of Magnesium-Strontium Doped Hydroxyapatite Nanocrystals: Towards the Production of 3D Biomimetic Bone Scaffolds. J. Biomed. Mater. Res. Part A 2020, 108, 633–644.
- Landi, E.; Guizzardi, S.; Papa, E.; Galli, C. Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Appl. Sci. 2021, 11, 4930.
- Wu, Q.; Zhang, X.; Wu, B.; Huang, W. Effects of Microwave Sintering on the Properties of Porous Hydroxyapatite Scaffolds. Ceram. Int. 2013, 39, 2389–2395.
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D Extrusion Bioprinting. Nat. Rev. Methods Primers 2021, 1, 75.
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D Printed Bone Tissue Regenerative PLA/HA Scaffolds with Comprehensive Performance Optimizations. Mater. Des. 2021, 201, 109490.
- Dukle, A.; Murugan, D.; Nathanael, A.J.; Rangasamy, L.; Oh, T.-H. Can 3D-Printed Bioactive Glasses Be the Future of Bone Tissue Engineering? Polymers 2022, 14, 1627.
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192.
- Wang, W.; Zhang, B.; Zhao, L.; Li, M.; Han, Y.; Wang, L.; Zhang, Z.; Li, J.; Zhou, C.; Liu, L. Fabrication and Properties of PLA/Nano-HA Composite Scaffolds with Balanced Mechanical Properties and Biological Functions for Bone Tissue Engineering Application. Nanotechnol. Rev. 2021, 10, 1359–1373.
- Tampieri, A.; Ruffini, A.; Ballardini, A.; Montesi, M.; Panseri, S.; Salamanna, F.; Fini, M.; Sprio, S. Heterogeneous Chemistry in the 3-D State: An Original Approach to Generate Bioactive, Mechanically-Competent Bone Scaffolds. Biomater. Sci. 2019, 7, 307–321.
- Šupová, M. Problem of Hydroxyapatite Dispersion in Polymer Matrices: A Review. J. Mater. Sci. Mater. Med. 2009, 20, 1201–1213.
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249.
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227.
- Tampieri, A.; Iafisco, M.; Sprio, S.; Ruffini, A.; Panseri, S.; Montesi, M.; Adamiano, A.; Sandri, M. Hydroxyapatite: From Nanocrystals to Hybrid Nanocomposites for Regenerative Medicine. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016.
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A.; Ghobrial, M.; Nath, D.; Morgano, S.M. Functional Role of Inorganic Trace Elements in Dentin Apatite Tissue—Part 1: Mg, Sr, Zn, and Fe. J. Trace Elem. Med. Biol. 2022, 71, 126932.
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface Phenomena Enhancing the Antibacterial and Osteogenic Ability of Nanocrystalline Hydroxyapatite, Activated by Multiple-Ion Doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959.
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. BioMed Res. Int. 2013, 2013, 929748.
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170.
- Shellis, R.P.R.; Featherstone, J.D.B.; Lussi, A. Understanding the Chemistry of Dental Erosion. Erosive Tooth Wear Diagn. Ther. 2012, 25, 163–179.
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016.
- Porter, A.; Patel, N.; Brooks, R.; Best, S.; Rushton, N.; Bonfield, W. Effect of Carbonate Substitution on the Ultrastructural Characteristics of Hydroxyapatite Implants. J. Mater. Sci. Mater. Med. 2005, 16, 899–907.
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate Substituted Hydroxyapatite: Resorption by Osteoclasts Modifies the Osteoblastic Response. J. Biomed. Mater. Res. Part A 2009, 90A, 217–224.
- Da Silva, L.M.; Tavares, D.d.S.; dos Santos, E.A. Isolating the Effects of Mg2+, Mn2+ and Sr2+ Ions on Osteoblast Behavior from Those Caused by Hydroxyapatite Transformation. Mater. Res. 2020, 23, e20200083.
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of Polycaprolactone on Alendronate Drug Release from Mg-Doped Hydroxyapatite Coating on Titanium. Mater. Sci. Eng. C 2018, 88, 166–171.
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, S.; Martra, G. Surface Characteristics of Nanocrystalline Apatites: Effect of Mg Surface Enrichment on Morphology, Surface Hydration Species, and Cationic Environments. Langmuir 2009, 25, 5647–5654.
- Brett, E.; Flacco, J.; Blackshear, C.; Longaker, M.T.; Wan, D.C. Biomimetics of Bone Implants: The Regenerative Road. BioRes. Open Access 2017, 6, 1–6.
- Verberckmoes, S.C.; Behets, G.J.; Oste, L.; Bervoets, A.R.; Lamberts, L.V.; Drakopoulos, M.; Somogyi, A.; Cool, P.; Dorriné, W.; de Broe, M.E.; et al. Effects of Strontium on the Physicochemical Characteristics of Hydroxyapatite. Calcif. Tissue Int. 2004, 75, 405–415.
- Ruffini, A.; Sandri, M.; Dapporto, M.; Campodoni, E.; Tampieri, A.; Sprio, S. Nature-Inspired Unconventional Approaches to Develop 3D Bioceramic Scaffolds with Enhanced Regenerative Ability. Biomedicines 2021, 9, 916.
- Kourkoumelis, N. Osteoporosis and Strontium-Substituted Hydroxyapatites. Ann. Transl. Med. 2016, 4, S10.
- Curran, D.J.; Fleming, T.J.; Towler, M.R.; Hampshire, S. Mechanical Parameters of Strontium Doped Hydroxyapatite Sintered Using Microwave and Conventional Methods. J. Mech. Behav. Biomed. Mater. 2011, 4, 2063–2073.
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, Biological, and Antibacterial Characteristics of Plasma-Sprayed (Sr,Zn) Substituted Hydroxyapatite Coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366.
- Chetty, A.; du Preez, I.; Marei, M.; Kamary, Y.E.; Moussa, R.M. Synthesis, Properties and Applications of Hydroxyapatite. In Hydroxyapatite: Synthesis, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 91–132.
- Dorozhkin, S.V. Dissolution Mechanism of Calcium Apatites in Acids: A Review of Literature. World J. Methodol. 2012, 2, 1.
- Dorozhkin, S.V. Inorganic Chemistry of the Dissolution Phenomenon: The Dissolution Mechanism of Calcium Apatites at the Atomic (Ionic) Level. Comments Inorg. Chem. 1999, 20, 285–299.
- Landi, E.; Sprio, S.; Sandri, M.; Celotti, G.; Tampieri, A. Development of Sr and CO3 Co-Substituted Hydroxyapatites for Biomedical Applications. Acta Biomater. 2008, 4, 656–663.
- Tampieri, A.; Celotti, G.C.; Landi, E.; Sandri, M. Magnesium Doped Hydroxyapatite: Synthesis and Characterization. Key Eng. Mater. 2004, 264–268, 2051–2054.
- Ziani, S.; Meski, S.; Khireddine, H. Characterization of Magnesium-Doped Hydroxyapatite Prepared by Sol-Gel Process. Int. J. Appl. Ceram. Technol. 2014, 11, 83–91.
- Arul, K.T.; Ramya, J.R.; Bhalerao, G.M.; Kalkura, S.N. Physicochemical Characterization of the Superhydrophilic, Magnesium and Silver Ions Co-Incorporated Nanocrystalline Hydroxyapatite, Synthesized by Microwave Processing. Ceram. Int. 2014, 40, 13771–13779.
- Zhu, H.; Guo, D.; Sun, L.; Li, H.; Hanaor, D.A.H.; Schmidt, F.; Xu, K. Nanostructural Insights into the Dissolution Behavior of Sr-Doped Hydroxyapatite. J. Eur. Ceram. Soc. 2018, 38, 5554–5562.
- Vukomanovic, M.; Gazvoda, L.; Anicic, N.; Rubert, M.; Suvorov, D.; Müller, R.; Hofmann, S. Multi-Doped Apatite: Strontium, Magnesium, Gallium and Zinc Ions Synergistically Affect Osteogenic Stimulation in Human Mesenchymal Cells Important for Bone Tissue Engineering. Biomater. Adv. 2022, 140, 213051.
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic Magnesium–Carbonate-Apatite Nanocrystals Endowed with Strontium Ions as Anti-Osteoporotic Trigger. Mater. Sci. Eng. C 2014, 35, 212–219.
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New Hydroxyapatite Nanophases with Enhanced Osteogenic and Anti-Bacterial Activity. J. Biomed. Mater. Res. Part A 2018, 106, 521–530.
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic Magnetism and Hyperthermia in Bioactive Fe-Doped Hydroxyapatite. Acta Biomater. 2012, 8, 843–851.
- Sprio, S.; Tampieri, A.; Landi, E.; Sandri, M.; Martorana, S.; Celotti, G.; Logroscino, G. Physico-Chemical Properties and Solubility Behaviour of Multi-Substituted Hydroxyapatite Powders Containing Silicon. Mater. Sci. Eng. C 2008, 28, 179–187.
- di Luca, A.; Longoni, A.; Criscenti, G.; Mota, C.; van Blitterswijk, C.; Moroni, L. Toward Mimicking the Bone Structure: Design of Novel Hierarchical Scaffolds with a Tailored Radial Porosity Gradient. Biofabrication 2016, 8, 045007.
- Chang, B.-S.; Lee, C.-K.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at Porous Hydroxyapatite with Various Pore Configurations. Biomaterials 2000, 21, 1291–1298.
- Elsheikh, M.; Kishida, R.; Hayashi, K.; Tsuchiya, A.; Shimabukuro, M.; Ishikawa, K. Effects of Pore Interconnectivity on Bone Regeneration in Carbonate Apatite Blocks. Regen. Biomater. 2022, 9, rbac010.
- Chu, T.-M.G.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in Vivo Performance of Hydroxyapatite Implants with Controlled Architectures. Biomaterials 2002, 23, 1283–1293.
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98.
- Rigo, E.C.S.; Boschi, A.O.; Yoshimoto, M.; Allegrini, S.; Konig, B.; Carbonari, M.J. Evaluation in Vitro and in Vivo of Biomimetic Hydroxyapatite Coated on Titanium Dental Implants. Mater. Sci. Eng. C 2004, 24, 647–651.
- Branemark, P.-I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410.
- Sprio, S.; Fricia, M.; Maddalena, G.F.; Nataloni, A.; Tampieri, A. Osteointegration in Cranial Bone Reconstruction: A Goal to Achieve. J. Appl. Biomater. Funct. Mater. 2016, 14, 470–476.
- Buckley, C.T.; O’Kelly, K.U. Fabrication and Characterization of a Porous Multidomain Hydroxyapatite Scaffold for Bone Tissue Engineering Investigations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 459–467.
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Filardo, G.; Kon, E.; Marcacci, M.; Tampieri, A. Composite Biomedical Foams for Engineering Bone Tissue. In Biomedical Foams for Tissue Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 249–280.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408.
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication Methods of Porous Metals for Use in Orthopaedic Applications. Biomaterials 2006, 27, 2651–2670.
- Zhang, J.; Jiang, Z.; Guo, H.; Sun, T.; Chen, A.; Zhou, Y.; He, Y. Function-Structure-Integrated Ti-HA Coatings on TiNbZr with Enhanced Mechanical Properties and Bioactivity Prepared by Spark Plasma Sintering. Vacuum 2021, 184, 109863.
- Liao, S.S.; Guan, K.; Cui, F.Z.; Shi, S.S.; Sun, T.S. Lumbar Spinal Fusion with a Mineralized Collagen Matrix and RhBMP-2 in a Rabbit Model. Spine 2003, 28, 1954–1960.
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically Biomimetic Bone Scaffold Materials: Nano-HA/Collagen/PLA Composite. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2004, 69, 158–165.
- Ma, J.; Wang, J.; Ai, X.; Zhang, S. Biomimetic Self-Assembly of Apatite Hybrid Materials: From a Single Molecular Template to Bi-/Multi-Molecular Templates. Biotechnol. Adv. 2014, 32, 744–760.
- Yao, Q.; Liu, S.; Zheng, W.; Chen, M.; Zhou, S.; Liao, M.; Huang, W.; Hu, Y.; Zhou, W. Formation of Poly(Ε-caprolactone)-embedded Bioactive Nanoparticles/Collagen Hierarchical Scaffolds with the Designed and Customized Porous Structures. J. Appl. Polym. Sci. 2022, 139, e52749.
- Jang, C.H.; Kim, W.; Kim, G. Effects of Fibrous Collagen/CDHA/HUCS Biocomposites on Bone Tissue Regeneration. Int. J. Biol. Macromol. 2021, 176, 479–489.
- Peroglio, M.; Gremillard, L.; Gauthier, C.; Chazeau, L.; Verrier, S.; Alini, M.; Chevalier, J. Mechanical Properties and Cytocompatibility of Poly(ε-Caprolactone)-Infiltrated Biphasic Calcium Phosphate Scaffolds with Bimodal Pore Distribution. Acta Biomater. 2010, 6, 4369–4379.
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803.
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. PEGylated Poly(Glycerol Sebacate)-Modified Calcium Phosphate Scaffolds with Desirable Mechanical Behavior and Enhanced Osteogenic Capacity. Acta Biomater. 2016, 44, 110–124.
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026.
- Sha, D.; Wu, Z.; Zhang, J.; Ma, Y.; Yang, Z.; Yuan, Y. Development of Modified and Multifunctional Poly(Glycerol Sebacate) (PGS)-Based Biomaterials for Biomedical Applications. Eur. Polym. J. 2021, 161, 110830.
- Rosenbalm, T.N.; Teruel, M.; Day, C.S.; Donati, G.L.; Morykwas, M.; Argenta, L.; Kuthirummal, N.; Levi-Polyachenko, N. Structural and Mechanical Characterization of Bioresorbable, Elastomeric Nanocomposites from Poly(Glycerol Sebacate)/Nanohydroxyapatite for Tissue Transport Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1366–1373.
- Ma, Y.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, J.; Shi, J.; Si, J.; Yuan, Y.; Liu, C. Direct Three-Dimensional Printing of a Highly Customized Freestanding Hyperelastic Bioscaffold for Complex Craniomaxillofacial Reconstruction. Chem. Eng. J. 2021, 411, 128541.
- Rodríguez, K.; Renneckar, S.; Gatenholm, P. Biomimetic Calcium Phosphate Crystal Mineralization on Electrospun Cellulose-Based Scaffolds. ACS Appl. Mater. Interfaces 2011, 3, 681–689.
- Choi, M.-O.; Kim, Y.-J. Effect of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Gelatin Ratios on the Characteristics of Biomimetic Composite Nanofibrous Scaffolds. Colloid Polym. Sci. 2018, 296, 917–926.
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Planell, J.A.; Castano, O. Electrospun Gelatin/Poly(ε-Caprolactone) Fibrous Scaffold Modified with Calcium Phosphate for Bone Tissue Engineering. Mater. Sci. Eng. C 2014, 44, 183–190.
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking Natural Bio-Mineralization Processes: A New Tool for Osteochondral Scaffold Development. Trends Biotechnol. 2011, 29, 526–535.
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.; Pugno, N.; Tampieri, A. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 3604.
- Frenkel, S.R.; Bradica, G.; Brekke, J.H.; Goldman, S.M.; Ieska, K.; Issack, P.; Bong, M.R.; Tian, H.; Gokhale, J.; Coutts, R.D.; et al. Regeneration of Articular Cartilage—Evaluation of Osteochondral Defect Repair in the Rabbit Using Multiphasic Implants. Osteoarthr. Cartil. 2005, 13, 798–807.
- Schek, R.M.; Taboas, J.M.; Segvich, S.J.; Hollister, S.J.; Krebsbach, P.H. Engineered Osteochondral Grafts Using Biphasic Composite Solid Free-Form Fabricated Scaffolds. Tissue Eng. 2004, 10, 1376–1385.
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural Origin Biodegradable Systems in Tissue Engineering and Regenerative Medicine: Present Status and Some Moving Trends. J. R. Soc. Interface 2007, 4, 999–1030.
- Jiang, C.-C.; Chiang, H.; Liao, C.-J.; Lin, Y.-J.; Kuo, T.-F.; Shieh, C.-S.; Huang, Y.-Y.; Tuan, R.S. Repair of Porcine Articular Cartilage Defect with a Biphasic Osteochondral Composite. J. Orthop. Res. 2007, 25, 1277–1290.
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138.
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115.
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of Different Crosslinking Agents on Hybrid Biomimetic Collagen-Hydroxyapatite Composites for Regenerative Medicine. Int. J. Biol. Macromol. 2018, 106, 739–748.
- Gajjeraman, S.; Narayanan, K.; Hao, J.; Qin, C.; George, A. Matrix Macromolecules in Hard Tissues Control the Nucleation and Hierarchical Assembly of Hydroxyapatite. J. Biol. Chem. 2007, 282, 1193–1204.
- Deng, Y.; Zhao, X.; Zhou, Y.; Zhu, P.; Zhang, L.; Wei, S. In Vitro Growth of Bioactive Nanostructured Apatites via Agar-Gelatin Hybrid Hydrogel. J. Biomed. Nanotechnol. 2013, 9, 1972–1983.
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science. 1993, 260, 920–926.
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly Osteochondral Regeneration in a Sheep Model Using a Novel Nano-Composite Multilayered Biomaterial. J. Orthop. Res. 2009, 28, 116–124.
- Kon, E.; Filardo, G.; di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical Results and MRI Evolution of a Nano-Composite Multilayered Biomaterial for Osteochondral Regeneration at 5 Years. Am. J. Sports Med. 2014, 42, 158–165.
- di Martino, A.; Kon, E.; Perdisa, F.; Sessa, A.; Filardo, G.; Neri, M.P.; Bragonzoni, L.; Marcacci, M. Surgical Treatment of Early Knee Osteoarthritis with a Cell-Free Osteochondral Scaffold: Results at 24 Months of Follow-Up. Injury 2015, 46, S33–S38.
- Berruto, M.; Delcogliano, M.; de Caro, F.; Carimati, G.; Uboldi, F.; Ferrua, P.; Ziveri, G.; de Biase, C.F. Treatment of Large Knee Osteochondral Lesions With a Biomimetic Scaffold. Am. J. Sports Med. 2014, 42, 1607–1617.
- Filardo, G.; Kon, E.; di Martino, A.; Busacca, M.; Altadonna, G.; Marcacci, M. Treatment of Knee Osteochondritis Dissecans with a Cell-Free Biomimetic Osteochondral Scaffold. Am. J. Sports Med. 2013, 41, 1786–1793.
- Vallet-Regí, M.; Ragel, V.; Salinas, A.J. Microreview Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042.
- Gunawidjaja, P.N.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Stevensson, B.; Grins, J.; Vallet-Regí, M.; Edén, M. Biomimetic Apatite Mineralization Mechanisms of Mesoporous Bioactive Glasses as Probed by Multinuclear 31P, 29Si, 23Na and 13C Solid-State NMR. J. Phys. Chem. C 2010, 114, 19345–19356.
- de Aza, P.N.; de Aza, A.H.; Pena, P.; de Aza, S. Bioactive Glasses and Glass-Ceramics. Bol. Soc. Esp. Ceram. Vidr. 2007, 46, 45–55.
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510.
- Sumi, K.; Abe, T.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Awada, T.; Nakajima, K.; Ando, K.; Tanimoto, K. The Effect of Mesenchymal Stem Cells on Chemotaxis of Osteoclast Precursor Cells. J. Oral Sci. 2018, 60, 221–225.
- Gómez-Cerezo, N.; Casarrubios, L.; Morales, I.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of a Mesoporous Bioactive Glass on Osteoblasts, Osteoclasts and Macrophages. J. Colloid Interface Sci. 2018, 528, 309–320.
- Łączka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. Bioactivity and Osteoinductivity of Glasses and Glassceramics and Their Material Determinants. Ceram. Int. 2016, 42, 14313–14325.
- Groh, D.; Döhler, F.; Brauer, D.S. Bioactive Glasses with Improved Processing. Part 1. Thermal Properties, Ion Release and Apatite Formation. Acta Biomater. 2014, 10, 4465–4473.
- Douglas, T.E.L.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.G.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable Self-Gelling Composites for Bone Tissue Engineering Based on Gellan Gum Hydrogel Enriched with Different Bioglasses. Biomed. Mater. 2014, 9, 045014.
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers 2018, 10, 381.
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and Evaluation of Chitosan/Chondroitin Sulfate/Nano-Bioglass Based Composite Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 817–830.
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Porous Poly(α-Hydroxyacid)/Bioglass® Composite Scaffolds for Bone Tissue Engineering. I: Preparation and in Vitro Characterisation. Biomaterials 2004, 25, 4185–4194.
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less Harmful Acidic Degradation of Poly(Lactic-Co-Glycolic Acid) Bone Tissue Engineering Scaffolds through Titania Nanoparticle Addition. Int. J. Nanomed. 2006, 1, 541–545.
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Production and Characterization of New Calcium Phosphate Bone Cements in the CaHPO4-Alpha-Ca3(PO4)2 System: PH, Workability and Setting Times. J. Mater. Sci. Mater. Med. 1999, 10, 223–230.
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049.
- Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A. Effect of the Particle Size on the Micro and Nanostructural Features of a Calcium Phosphate Cement: A Kinetic Analysis. Biomaterials 2004, 25, 3453–3462.
- Shimogoryo, R.; Eguro, T.; Kimura, E.; Maruta, M.; Matsuya, S.; Ishikawa, K. Effects of Added Mannitol on the Setting Reaction and Mechanical Strength of Apatite Cement. Dent. Mater. J. 2009, 28, 627–633.
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922.
- Patil, S.; Montgomery, R. Management of Complex Tibial and Femoral Nonunion Using the Ilizarov Technique, and Its Cost Implications. J. Bone Jt. Surgery. Br. Vol. 2006, 88-B, 928–932.
- Lozada-Gallegos, A.R.; Letechipia-Moreno, J.; Palma-Lara, I.; Montero, A.A.; Rodríguez, G.; Castro-Muñozledo, F.; Cornejo-Cortés, M.A.; Juárez-Mosqueda, M.L. Development of a Bone Nonunion in a Noncritical Segmental Tibia Defect Model in Sheep Utilizing Interlocking Nail as an Internal Fixation System. J. Surg. Res. 2013, 183, 620–628.
- Pilia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. BioMed Res. Int. 2013, 2013, 458253.
- Sprio, S.; Panseri, S.; Montesi, M.; Dapporto, M.; Ruffini, A.; Dozio, S.M.; Cavuoto, R.; Misseroni, D.; Paggi, M.; Bigoni, D.; et al. Hierarchical Porosity Inherited by Natural Sources Affects the Mechanical and Biological Behaviour of Bone Scaffolds. J. Eur. Ceram. Soc. 2020, 40, 1717–1727.
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729.
- Baino, F.; Ferraris, M. Learning from Nature: Using Bioinspired Approaches and Natural Materials to Make Porous Bioceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 507–520.
- Fan, T.-X.; Chow, S.-K.; Zhang, D. Biomorphic Mineralization: From Biology to Materials. Prog. Mater. Sci. 2009, 54, 542–659.
- White, R.A.; Weber, J.N.; White, E.W. Replamineform: A New Process for Preparing Porous Ceramic, Metal, and Polymer Prosthetic Materials. Science 1972, 176, 922–924.
