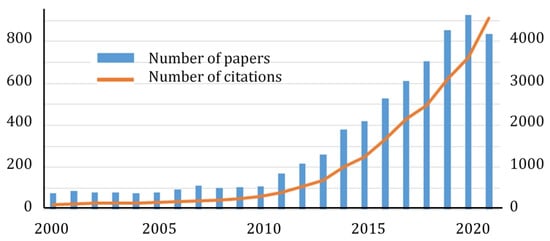
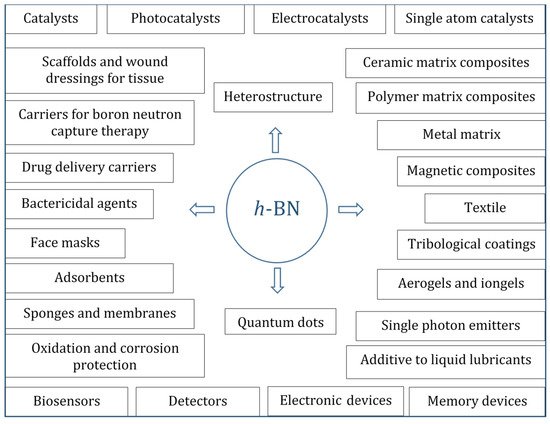
Due to its unique physical, chemical, and mechanical properties, such as a low specific density, large specific surface area, excellent thermal stability, oxidation resistance, low friction, good dispersion stability, enhanced adsorbing capacity, large interlayer shear force, and wide bandgap, hexagonal boron nitride (h-BN) nanostructures are of great interest in many fields. These include, but are not limited to, (i) heterogeneous catalysts, (ii) promising nanocarriers for targeted drug delivery to tumor cells and nanoparticles containing therapeutic agents to fight bacterial and fungal infections, (iii) reinforcing phases in metal, ceramics, and polymer matrix composites, (iv) additives to liquid lubricants, (v) substrates for surface enhanced Raman spectroscopy, (vi) agents for boron neutron capture therapy, (vii) water purifiers, (viii) gas and biological sensors, and (ix) quantum dots, single photon emitters, and heterostructures for electronic, plasmonic, optical, optoelectronic, semiconductor, and magnetic devices. BNNPs and BN-based nanohybrids exhibit antibacterial and antifungal activity.
- hexagonal BN
- nanostructures
- nanohybrids
- fabrication
- application
1. Introduction
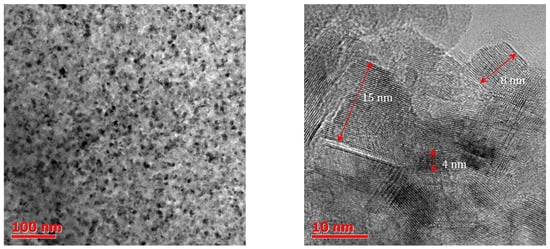
2. Fabrication and Surface Functionalization
2.1. Bottom-Up Approach
2.2. Top-Down Approach
2.2.1. Microfluidization
2.2.2. Ball Milling
3. Catalysts
3.1. Heterogeneous and Homogeneous Catalysts
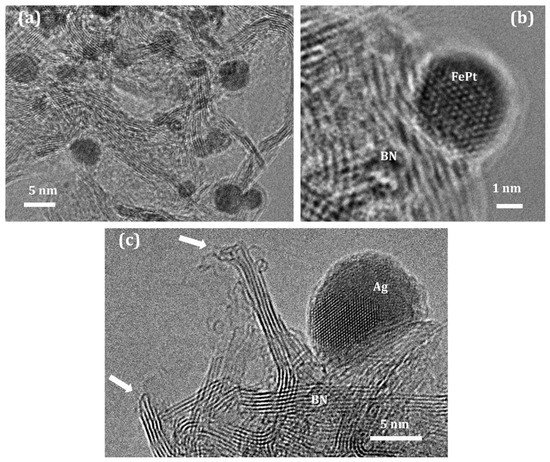
3.2. Photocatalysts and Electrocatalysts
4. Materials for Biomedicine and Improvement of Quality of Life
4.1. Biocompatibility and Dose-Dependent Toxicity
4.2. Antibacterial and Antifungal Activity
| Material | BNNP Content (%) | Pathogens | Ref. |
|---|---|---|---|
| PNMPy-BNNPs | 10.0 | E. coli, S. aureus, P. aeruginosa, E. faecalis | [107] |
| LDPE-BNNPs | 5.0–20.0 | E. coli, S. aureus, P. aeruginosa, S. epidermidis |
[108] |
| PHA/CH-BNNPs | 0.1–1.0 | E. coli K1 Methicillin-resistant S. aureus |
[109] |
| QAC-BNNPs-PP | 3.0–10.0 | E. coli Carolina #155065A S. aureus Carolina #155556 |
[110] |
| CEL-BNNPs | 1.0–3.0 | E. coli K12 (ATCC 29425) S. epidermidis ATCC 49461 |
[111] |
| MIC of BN (mg/mL) | |||
| BNNPs | 15 | Multidrug resistant E. coli (12 strains) | [112] |
| BNNPs | 1.62 | S. mutans 3.3 | [113] |
| 400 | S. mutans ATTC 25175 | ||
| 400 | S. pasteuri M3 | ||
| 3.25 | Candida sp. M25 | ||
| BNNPs | 256 | E. coli | [114] |
| 128 | B. cereus | ||
| 128 | S. aureus | ||
| 128 | E. hirae | ||
| 128 | P. aeruginosa | ||
| 256 | L. pneumophila subsp. pneumophiia | ||
| 256 | C. albicans | ||
| BNNSs | 100 | E. coli DH5α | [115] |
4.3. Drug Delivery
4.4. Boron Neutron Capture Therapy
4.5. Tissue Engineering
This entry is adapted from the peer-reviewed paper 10.3390/nano12162810
References
- Shtansky, D.V.; Tsuda, O.; Ikuhara, Y.; Yoshida, T. Crystallography and Structural Evolution of Cubic Boron Nitride Films during Bias Sputter Deposition. Acta Mater. 2000, 48, 3745–3759.
- Sponza, L.; Amara, H.; Attaccalite, C.; Latil, S.; Galvani, T.; Paleari, F.; Wirtz, L.; Ducastelle, F. Direct and Indirect Excitons in Boron Nitride Polymorphs: A Story of Atomic Configuration and Electronic Correlation. Phys. Rev. B 2018, 98, 125206.
- Warner, J.H.; Rümmeli, M.H.; Bachmatiuk, A.; Büchner, B. Atomic Resolution Imaging and Topography of Boron Nitride Sheets Produced by Chemical Exfoliation. ACS Nano 2010, 4, 1299–1304.
- Sutter, P.; Lahiri, J.; Zahl, P.; Wang, B.; Sutter, E. Scalable Synthesis of Uniform Few-Layer Hexagonal Boron Nitride Dielectric Films. Nano Lett. 2013, 13, 276–281.
- Park, H.J.; Cha, J.; Choi, M.; Kim, J.H.; Tay, R.Y.; Teo, E.H.T.; Park, N.; Hong, S.; Lee, Z. One-Dimensional Hexagonal Boron Nitride Conducting Channel. Sci. Adv. 2020, 6, eaay4958.
- Cassabois, G.; Valvin, P.; Gil, B. Hexagonal Boron Nitride is an Indirect Bandgap Semiconductor. Nat. Photonics 2016, 10, 262–266.
- Yang, Y.; Song, Z.; Lu, G.; Zhang, Q.; Zhang, B.; Ni, B.; Wang, C.; Li, X.; Xie, X.; Gao, H.; et al. Intrinsic Toughening and Stable Crack Propagation in Hexagonal Boron Nitride. Nature 2021, 594, 57–61.
- Wagemann, E.; Wang, Y.; Das, S.; Mitra, S.K. On the Wetting Translucency of Hexagonal Boron Nitride. Phys. Chem. Chem. Phys. 2020, 22, 7710–7718.
- Duerloo, K.-A.N.; Ong, M.T.; Reed, E.J. Intrinsic Piezoelectricity in Two-Dimensional Materials. J. Phys. Chem. Lett. 2012, 3, 2871–2876.
- Ares, P.; Cea, T.; Holwill, M.; Wang, Y.B.; Roldán, R.; Guinea, F.; Andreeva, D.V.; Fumagalli, L.; Novoselov, K.S.; Woods, C.R. Piezoelectricity in Monolayer Hexagonal Boron Nitride. Adv. Mater. 2019, 32, 1905504.
- Kundalwal, S.I.; Choyal, V. Enhancing the Piezoelectric Properties of Boron Nitride Nanotubes through Defect Engineering. Phys. E 2021, 125, 114304.
- Xiao, Y.; Yu, H.; Wang, H.; Zhu, X.; Chen, L.; Gao, W.; Liu, G.; Yin, H. Defect Engineering of Hexagonal Boron Nitride Nanosheets via Hydrogen Plasma Irradiation. Appl. Surf. Sci. 2022, 593, 153386.
- Cretu, O.; Ishizuka, A.; Yanagisawa, K.; Ishizuka, K.; Kimoto, K. Atomic-Scale Electrical Field Mapping of Hexagonal Boron Nitride Defects. ACS Nano 2021, 15, 5316–5321.
- Balmain, W.H. Bemerkungen über die Bildung von Verbindungen des Bors und Siliciums mit Stickstoff und gewissen Metallen. J. Prakt. Chem. 1842, 27, 422–430.
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967.
- Yu, I.; Jo, Y.; Ko, J.; Moon, S.Y.; Ahn, S.Y.M.; Joo, Y. Highly Aligned Array of Heterostructured Polyflourene-Isolated Boron Nitride and Carbon Nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 12417–12424.
- Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Sukhorukova, I.V.; Firestein, K.L.; Steinman, A.E.; Shtansky, D.V.; Golberg, D. Growth of Spherical Boron Oxynitride Banoparticles with Smooth and Petalled Surfaces during Chemical Vapor Deposition Process. CrystEngComm 2016, 18, 6689–6699.
- Le, T.-H.; Oh, Y.; Kim, H.; Yoon, H. Exfoliation of 2D Materials for Energy and Environmental Applications. Chem. Eur. J. 2022, 26, 6360–6401.
- Gautam, C.; Chelliah, S. Methods of Hexagonal Boron Nitride Exfoliation and Its Functionalization: Covalent and Non-Covalent Approaches. RSC Adv. 2021, 11, 31284–31327.
- Juma, I.G.; Kim, G.; Jariwala, D.; Behura, S.K. Direct Growth of Hexagonal Boron Nitride on Non-Metallic Substrates and Its Heterostructures with Graphene. iScience 2021, 24, 103374–103393.
- Shen, X.; Zheng, Q.; Kim, J.-K. Rational Design of Two-Dimensional Nanofillers for Polymer Nanocomposites toward Multifunctional Applications. Prog. Mater. Sci. 2021, 115, 100708–100773.
- Meziani, M.J.; Sheriff, K.; Parajuli, P.; Priego, P.; Bhattacharya, S.; Rao, A.M.; Quimby, J.L.; Qiao, R.; Wang, P.; Hwu, S.-J.; et al. Advances in Studies of Boron Nitride Nanosheets and Nanocomposites for Thermal Transport and Related Applications. ChemPhysChem 2022, 23, e202100645–e202100668.
- Revabhai, P.M.; Singhal, R.K.; Basu, H.; Kailasa, S.K. Progress on Boron Nitride Nanostructure Materials: Properties, Synthesis and Applications in Hydrogen Storage and Analytical Chemistry. J. Nanostruct. Chem. 2022.
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589.
- Yang, Y.; Peng, Y.; Saleem, M.F.; Chen, Z.; Sun, W. Hexagonal Boron Nitride on III–V Compounds: A Review of the Synthesis and Applications. Materials 2022, 15, 4396.
- Yasnó, J.; Kiminami, R.H.G.A. Short Time Reaction Synthesis of Nano-Hexagonal Boron Nitride. Adv. Powder Technol. 2020, 31, 4436–4443.
- Matveev, A.T.; Permyakova, E.S.; Kovalskii, A.M.; Leibo, D.; Shchetinin, I.V.; Maslakov, K.I.; Golberg, D.V.; Shtansky, D.V.; Konopatsky, A.S. New Insights into Synthesis of Nanocrystalline Hexagonal BN. Ceram. Int. 2020, 46, 19866–19872.
- Köken, D.; Sungur, P.; Cebeci, H.; Cebeci, F.C. Revealing the Effect of Sulfur Compounds for Low-Temperature Synthesis of Boron Nitride Nanotubes from Boron Minerals. ACS Appl. Nano Mater. 2022, 5, 2137–2146.
- Silva-Santos, S.D.; Impellizzeri, A.; Aguiar, A.L.; Journet, C.; Dalverny, C.; Toury, B.; De Sousa, J.M.; Ewels, C.P.; San-Miguel, A. High Pressure in Boron Nitride Nanotubes for Kirigami Nanoribbon Elaboration. J. Phys. Chem. C 2021, 125, 11440–11453.
- Pham, T.; Stonemeyer, S.; Marquez, J.; Long, H.; Gillbert, M.; Worsley, M.; Zettl, A. One-Step Conversion of Graphite to Crinkled Boron Nitride Nanofoams for Hydrophobic Liquid Absorption. ACS Appl. Nano Mater. 2021, 4, 3500–3507.
- Matsoso, B.; Vuillet-a-Ciles, V.; Bois, L.; Toury, B.; Journet, C. Improving Formation Conditions and Properties of hBN Nanosheets through BaF2-Assisted Polymer Derived Ceramics (PDCs) Technique. Nanomaterials 2020, 10, 443.
- Liu, F.; Han, R.; Naficy, S.; Casillas, G.; Sun, X.; Huang, Z. Few-Layered Boron Nitride Nanosheets for Strengthening Polyurethane Hydrogels. ACS Appl. Nano Mater. 2021, 4, 7988–7994.
- Kondo, D.; Kataoka, M.; Hayashi, K.; Sato, S. Few-Layer Hexagonal Boron Nitride Synthesized by Chemical Vapor Deposition and Its Insulating Properties. Nano Express 2021, 2, 030001–030007.
- Shi, Z.; Wang, X.; Li, Q.; Yang, P.; Lu, G.; Jiang, R.; Wang, H.; Zhang, C.; Cong, C.; Liu, Z.; et al. Vapor–Liquid–Solid Growth of Large-Area Multilayer Hexagonal Boron Nitride on Dielectric Substrates. Nat. Comm. 2020, 11, 849–857.
- Li, Y.; Garnier, V.; Steyer, P.; Journet, C.; Toury, B. Millimeter-Scale Hexagonal Boron Nitride Single Crystals for Nanosheet Generation. ACS Appl. Nano Mater. 2020, 3, 1508–1515.
- Li, J.; Wang, J.; Zhang, X.; Elias, C.; Ye, G.; Evans, D.; Eda, G.; Redwing, J.M.; Cassabois, G.; Gil, B.; et al. Hexagonal Boron Nitride Crystal Growth from Iron, a Single Component Flux. ACS Nano 2021, 15, 7032–7039.
- Bansal, A.; Hilse, M.; Huet, B.; Wang, K.; Kozhakhmetov, A.; Kim, J.H.; Bachu, S.; Alem, N.; Collazo, R.; Robinson, J.A.; et al. Substrate Modification During Chemical Vapor Deposition of hBN on Sapphire. ACS Appl. Mater. Interfaces 2021, 13, 54516–54526.
- Chen, J.; Wang, G.; Meng, J.; Cheng, Y.; Yin, Z.; Tian, Y.; Huang, J.; Zhang, S.; Wu, J.; Zhang, X. Low-Temperature Direct Growth of Few-Layer Hexagonal Boron Nitride on Catalyst-Free Sapphire Substrates. ACS Appl. Mater. Interfaces 2022, 14, 7004–7011.
- Singhal, R.; Echeverria, E.; McIlroy, D.N.; Singh, R.N. Synthesis of Hexagonal Boron Nitride Films on Silicon and Sapphire Substrates by Low-Pressure Chemical Vapor Deposition. Thin Solid Film. 2021, 733, 138812.
- Gigliotti, J.; Li, X.; Sundaram, S.; Deniz, D.; Prudkovskiy, V.; Turmaud, J.-P.; Hu, Y.; Hu, Y.; Fossard, F.; Meérot, J.-S.; et al. Highly Ordered Boron Nitride/Epigraphene Epitaxial Films on Silicon Carbide by Lateral Epitaxial Deposition. ACS Nano 2020, 14, 12962–12971.
- Arjmandi-Tash, H. In Situ Growth of Graphene on Hexagonal Boron Nitride for Electronic Transport Applications. J. Mater. Chem. C 2020, 8, 380–386.
- Yan, Q.; Dai, W.; Gao, J.; Tan, X.; Lv, L.; Ying, J.; Lu, X.; Lu, J.; Yao, Y.; Wei, Q.; et al. Ultrahigh-Aspect-Ratio Boron Nitride Nanosheets Leading to Superhigh In-Plane Thermal Conductivity of Foldable Heat Spreader. ACS Nano 2021, 15, 6489–6498.
- Li, S.; Lu, X.; Lou, Y.; Liu, K.; Zou, B. The Synthesis and Characterization of h-BN Nanosheets with High Yield and Crystallinity. ACS Omega 2021, 6, 27814–27822.
- Wang, Z.-G.; Wei, X.; Bai, M.-H.; Lei, J.; Xu, L.; Huang, H.-D.; Du, J.; Dai, K.; Xu, J.-Z.; Li, Z.-M. Green Production of Covalently Functionalized Boron Nitride Nanosheets Via Saccharide-Assisted Mechanochemical Exfoliation. ACS Sustain. Chem. Eng. 2021, 9, 11155–11162.
- Huang, J.; E, S.; Li, J.; Jia, F.; Ma, Q.; Hua, L.; Lu, Z. Ball-Milling Exfoliation of Hexagonal Boron Nitride in Viscous Hydroxyethyl Cellulose for Producing Nanosheet Films as Thermal Interface Materials. ACS Appl. Nano Mater. 2021, 4, 13167–13175.
- Yusupov, K.U.; Corthay, S.; Bondarev, A.V.; Kovalskii, A.M.; Matveev, A.T.; Arkhipov, D.; Golberg, D.; Shtansky, D.V. Spark Plasma Sintered Al-based Composites Reinforced with BN Nanosheets Exfoliated under Ball Milling in Ethylene Glycol. Mater. Sci. Eng. A 2019, 745, 74–81.
- Ding, J.-H.; Zhao, H.-R.; Yu, H.-B. High-Yield Synthesis of Extremely High Concentrated and Few-Layered Boron Nitride Nanosheet Dispersions. 2D Mater. 2018, 5, 045015–045033.
- Du, Y.; Zhang, Y.; Zhang, R.; Lin, S. Synthesis of Ultrathin Functional Boron Nitride Nanosheets and Their Application in Anticorrosion. ACS Appl. Nano Mater. 2021, 4, 11088–11096.
- Kovalskii, A.M.; Matveev, A.T.; Popov, Z.I.; Volkov, I.N.; Sukhanova, E.V.; Lytkina, A.A.; Yaroslavtsev, A.B.; Konopatsky, A.S.; Leybo, D.V.; Bondarev, A.V.; et al. (Ni,Cu)/Hexagonal BN Nanohybrids—New Efficient Catalysts for Methanol Steam Reforming and Carbon Monoxide Oxidation. Chem. Eng. J. 2020, 395, 125109.
- Konopatsky, A.S.; Firestein, K.L.; Leybo, D.V.; Sukhanova, E.V.; Popov, Z.I.; Fang, X.; Manakhov, A.M.; Kovalskii, A.M.; Matveev, A.T.; Shtansky, D.V.; et al. Structural Evolution of Ag/BN Hybrids via a Polyol-Assisted Fabrication Process and Their Catalytic Activity in CO oxidation. Catal. Sci. Technol. 2019, 9, 6460–6470.
- Grant, J.T.; Carrero, C.A.; Goeltl, F.; Venegas, J.; Mueller, P.; Burt, S.P.; Specht, S.E.; McDermott, W.P.; Chieregato, A.; Hermans, I. Selective Oxidative Dehydrogenation of Propane to Propene Using Boron Nitride Catalysts. Science 2016, 354, 1570–1573.
- Venegas, J.M.; Hermans, I. The Influence of Reactor Parameters on the Boron Nitride-Catalyzed Oxidative Dehydrogenation of Propane. Org. Process Res. Dev. 2018, 22, 1644–1652.
- McDermott, W.P.; Venegas, J.; Hermans, I. Selective Oxidative Cracking of N-butane to Light Olefins over Hexagonal Boron Nitride with Limited Formation of COx. ChemSusChem 2020, 13, 152–158.
- Kraus, P.; Lindstedt, R.P. It’s a Gas: Oxidative Dehydrogenation of Propane over Boron Nitride Catalysts. J. Phys. Chem. C 2021, 125, 5623–5634.
- Du, M.; Liu, Q.; Huang, C.; Qiu, X. One-Step Synthesis of Magnetically Recyclable Core–Shell Nanocatalysts for Catalytic Reduction of Nitroarenes. RSC Adv. 2017, 7, 35451–35459.
- Chen, N.; Zhu, Z.; Su, T.; Liao, W.; Deng, C.; Ren, W.; Zhao, Y.; Lü, H. Catalytic Hydrogenolysis of Hydroxymethylfurfural to Highly Selective 2,5-Dimethylfuran over FeCoNi/h-BN Catalyst. Chem. Eng. J. 2020, 381, 122755.
- Zhang, R.; Wang, L.; Yang, X.; Tao, Z.; Ren, X.; Lv, B. The Role of Surface NH Groups on the Selective Hydrogenation of Cinnamaldehyde over Co/BN Catalysts. Appl. Surf. Sci. 2019, 492, 736–745.
- Ji, H.; Ju, H.; Lan, R.; Wu, P.; Sun, J.; Chao, Y.; Xun, S.; Zhu, W.; Li, H. Phosphomolybdic Acid Immobilized on Ionic Liquid-Modified Hexagonal Boron Nitride for Oxidative Desulfurization of Fuel. RSC Adv. 2017, 7, 54266–54276.
- Cheng, S.-Q.; Weng, X.-F.; Wang, Q.-N.; Zhou, B.-C.; Li, W.-C.; Li, M.-R.; He, L.; Wang, D.-Q.; Lu, A.-H. Defect-Rich BN-Supported Cu with Superior Dispersion for Ethanol Conversion to Aldehyde and Hydrogen. Chin. J. Catal. 2022, 43, 1092–1100.
- Konopatsky, A.S.; Leybo, D.V.; Firestein, K.L.; Chepkasov, I.V.; Popov, Z.I.; Permyakova, E.S.; Volkov, I.N.; Kovalskii, A.M.; Matveev, A.T.; Shtansky, D.V.; et al. Polyol Synthesis of Ag/BN Nanohybrids and Their Catalytic Stability in CO Oxidation Reaction. ChemCatChem 2020, 12, 1691–1698.
- Wang, L.; Wang, Y.; Zhang, C.-W.; Wen, J.; Weng, X.; Shi, L. A Boron Nitride Nanosheet-Supported Pt/Cu Cluster as a High-Efficiency Catalyst for Propane Dehydrogenation. Catal. Sci. Technol. 2020, 10, 1248–1255.
- Li, L.; Liu, X.; He, H.; Zhang, N.; Liu, Z.; Zhang, G. A Novel Two-Dimensional MgO-h-BN Nanomaterial Supported Pd Catalyst for CO Oxidation Reaction. Catal. Today 2019, 332, 214–221.
- Lu, M.; Zhang, X.; Deng, J.; Kuboon, S.; Faungnawakij, K.; Xiao, S.; Zhang, D. Coking-Resistant Dry Reforming of Methane over BN–Nanoceria Interface-Confined Ni Catalysts. Catal. Sci. Technol. 2020, 10, 4237–4244.
- Kovalskii, A.M.; Volkov, I.N.; Evdokimenko, N.D.; Leybo, D.V.; Chepkasov, I.V.; Popov, Z.I.; Matveev, A.T.; Manahov, A.M.; Permyakova, E.S.; Konopatsky, A.S.; et al. (Au and Pt)/Hexagonal BN Nanohybrids in Carbon Monoxide Oxidation and Carbon Dioxide Hydrogenation Reactions. Appl. Catal. B-Environ. 2022, 303, 120891.
- Konopatsky, A.S.; Firestein, K.L.; Evdokimenko, N.D.; Baidyshev, V.S.; Chepkasov, I.V.; Popov, Z.I.; Matveev, A.T.; Shetinin, I.V.; Leybo, D.V.; Volkov, I.N.; et al. Microstructure and Catalytic Properties of Fe3O4/BN, Fe3O4(Pt)/BN, and FePt/BN Nanohybrids in CO2 Hydrogenation Reaction: Experimental and Theoretical Insights. J. Catal. 2021, 402, 130–142.
- Sheng, J.; Li, W.-C.; Lu, W.-D.; Yan, B.; Qiu, B.; Gao, X.-Q.; Zhang, R.-P.; Zhou, S.-Z.; Lu, A.-H. Preparation of Oxygen Reactivity-Tuned FeOx/BN Catalyst for Selectively Oxidative Dehydrogenation of Ethylbenzene to Styrene. Appl. Catal. B-Environ. 2022, 305, 121070.
- Wang, Y.; Wang, J.; Zheng, P.; Sun, C.; Luo, J.; Xie, X. Boosting Selectivity and Stability on Pt/BN Catalysts for Propane Dehydrogenation via Calcination & Reduction-Mediated Strong Metal-Support Interaction. J. Energy Chem. 2022, 67, 451–457.
- Wang, X.; Zhang, C.; Chang, Q.; Wang, L.; Lv, B.; Xu, J.; Xiang, H.; Yang, Y.; Li, Y. Enhanced Fischer-Tropsch Synthesis Performances of Fe/h-BN Catalysts by Cu and Mn. Catal. Today 2020, 343, 91–100.
- Peinado, C.; Liuzzi, D.; Ladera-Gallardo, R.M.; Retuerto, M.; Ojeda, M.; Peña, M.A.; Rojas, S. Effects of Support and Reaction Pressure for the Synthesis of Dimethyl Ether over Heteropolyacid Catalysts. Sci. Rep. 2020, 10, 8551.
- Li, X.; Lin, B.; Li, H.; Yu, Q.; Ge, Y.; Jin, X.; Liu, X.; Zhou, Y.; Xiao, J. Carbon Doped Hexagonal BN as a Highly Efficient Metal-Free Base Catalyst for Knoevenagel Condensation Reaction. Appl. Catal. B-Environ. 2018, 239, 254–259.
- Wang, X.; Liu, Z.; Shi, X.; Jia, Y.; Zhu, G.; Peng, J.; Wang, Q. Optical and Photocatalytic Characteristics of Se-Doped h-Boron Nitride: Experimental Assessments and DFT Calculations. J. Alloys Compd. 2022, 909, 164791.
- Wang, J.; Sun, C.; Xia, W.; Cao, Z.; Sheng, G.; Xie, X. Pd/BN Catalysts for Highly Efficient Hydrogenation of Maleic Anhydride to Succinic Anhydride. Appl. Catal. A-Gen. 2022, 630, 118471.
- Wang, Y.; Chen, J.; Wang, L.; Weng, H.; Wu, Z.; Jiao, L.; Muroya, Y.; Yamashita, S.; Cheng, S.; Li, F.; et al. γ-Radiation Synthesis of Ultrasmall Noble Metal (Pd, Au, Pt) Nanoparticles Embedded on Boron Nitride Nanosheets for High-Performance Catalysis. Ceram. Int. 2021, 47, 26963–26970.
- Cao, Z.; Bu, J.; Zhong, Z.; Sun, C.; Zhang, Q.; Wang, J.; Chen, S.; Xie, X. Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over BN-Supported Pt Catalysts at Room Temperature. Appl. Catal. A-Gen. 2019, 578, 105–115.
- Gao, M.; Jia, X.; Ma, J.; Fan, X.; Gao, J.; Xu, J. Self-Regulated Catalysis for the Selective Synthesis of Primary Amines from Carbonyl Compounds. Green Chem. 2021, 23, 7115–7121.
- Zhu, W.; Wu, Z.; Foo, G.S.; Gao, X.; Zhou, M.; Liu, B.; Veith, G.M.; Wu, P.; Browning, K.L.; Lee, H.N.; et al. Taming Interfacial Electronic Properties of Platinum Nanoparticles on Vacancy-Abundant Boron Nitride Nanosheets for Enhanced Catalysis. Nat. Comm. 2017, 8, 15291.
- Lu, L.-J.; Wu, P.-W.; He, J.; Hua, M.-Q.; Chao, Y.-H.; Yang, N.; Chen, L.-L.; Jiang, W.; Fan, L.; Ji, H.-B.; et al. N-Hydroxyphthalimide Anchored on Hexagonal Boron Nitride as a Metal-Free Heterogeneous Catalyst for Deep Oxidative Desulfurization. Pet. Sci. 2021, 19, 1382–1389.
- Rana, P.; Dixit, R.; Sharma, S.; Dutta, S.; Yadav, S.; Sharma, A.; Kaushik, B.; Rana, P.; Adholeya, A.; Sharma, R.K. Enhanced Catalysis through Structurally Modified Hybrid 2-D Boron Nitride Nanosheets Comprising of Complexed 2-Hydroxy-4-Methoxybenzophenone Motif. Sci. Rep. 2021, 11, 24429.
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysts. Nat. Rev. Chem. 2018, 2, 65–81.
- Kaiser, S.K.; Chen, Z.; Akl, D.F.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809.
- Lei, Y.; Pakhira, S.; Fujisawa, K.; Liu, H.; Guerrero-Bermea, C.; Zhang, T.; Dasgupta, A.; Martinez, L.M.; Singamaneni, S.R.; Wang, K.; et al. Low Temperature Activation of Inert Hexagonal Boron Nitride for Metal Deposition and Single Atom Catalysis. Mater. Today 2021, 51, 108–116.
- Li, Z.; Wei, W.; Li, H.; Li, S.; Leng, L.; Zhang, M.; Horton, J.H.; Wang, D.; Sun, W.; Guo, C.; et al. Low-Temperature Synthesis of Single Palladium Atoms Supported on Defective Hexagonal Boron Nitride Nanosheet for Chemoselective Hydrogenation of Cinnamaldehyde. ACS Nano 2021, 15, 10175–10184.
- Huang, X.; Akdim, O.; Douthwaite, M.; Wang, K.; Zhao, L.; Lewis, R.J.; Pattisson, S.; Daniel, I.T.; Miedziak, P.J.; Shaw, G.; et al. Au–Pd Separation Enhances Bimetallic Catalysis of Alcohol Oxidation. Nature 2022, 603, 271–275.
- Zhu, J.; Xu, W.; Chen, J.; Gan, Z.; Wang, X.; Zhou, J. Development of Core–Shell Structured Mo2 as Novel Microwave Catalysts for Highly Effective Direct Decomposition of H2S into H2 and S at Low Temperature. Catal. Sci. Technol. 2020, 10, 6769–6779.
- Erusappan, E.; Thiripuranthagan, S.; Durai, M.; Kumaravel, S.; Vembuli, T. Photocatalytic Performance of Visible Active Boron Nitride Supported ZnFe2O4 (ZnFe2O4/BN) Nanocomposites for the Removal of Aqueous Organic Pollutants. New J. Chem. 2020, 44, 7758–7770.
- Ren, K.; Dong, Y.; Chen, Y.; Shi, H. Bi2WO6 Nanosheets Assembled BN Quantum Dots: Enhanced Charge Separation and Photocatalytic Antibiotics Degradation. Colloids Surf. A 2022, 637, 128208.
- Yan, T.; Du, Z.; Wang, J.; Cai, H.; Bi, D.; Guo, Z.; Liu, Z.; Tang, C.; Fang, Y. Construction of 2D/2D Bi2WO6/BN Heterojunction for Effective Improvement on Photocatalytic Degradation of Tetracycline. J. Alloys Compd. 2022, 894, 162487.
- Li, W.; Wang, F.; Chu, X.; Dang, Y.; Liu, X.; Ma, T.; Li, J.; Wang, C. 3D Porous BN/RGO Skeleton Embedded by MoS2 Nanostructures for Simulated-Solar-Light Induced Hydrogen Production. Chem. Eng. J. 2022, 435, 132441.
- Jiang, C.; Zhang, M.; Dong, G.; Wei, T.; Feng, J.; Ren, Y.; Luan, T. Photocatalytic Nitrate Reduction by a Non-Metal Catalyst h-BN: Performance and Mechanism. Chem. Eng. J. 2022, 429, 132216.
- Haroon, H.; Wahid, M.; Majid, K. Metal−Organic Framework-Derived p-Type Cu3P/Hexagonal Boron Nitride Nanostructures for Photocatalytic Oxidative Coupling of Aryl Halides to Biphenyl Derivatives. ACS Appl. Nano Mater. 2022, 5, 2006–2017.
- Bao, Y.; Yan, W.; Sun, P.-P.; Seow, J.Z.Y.; Lua, S.K.; Lee, W.J.; Liang, Y.N.; Lim, T.-T.; Xu, Z.J.; Zhou, K.; et al. Unexpected Intrinsic Catalytic Function of Porous Boron Nitride Nanorods for Highly Efficient Peroxymonosulfate Activation in Water Treatment. ACS Appl. Mater. Interfaces 2022, 14, 18409–18419.
- Matveev, A.T.; Konopatsky, A.S.; Leybo, D.V.; Volkov, I.N.; Kovalskii, A.M.; Varlamova, L.A.; Sorokin, P.B.; Fang, X.; Shtansky, D.V. Amorphous MoSxOy/h-BNxOy Nanohybrids: Synthesis and Dye Photodegradation. Nanomaterials 2021, 11, 3232.
- Zhao, G.; Wang, A.; He, W.; Xing, Y.; Xu, X. 2D New Nonmetal Photocatalyst of Sulfur-Doped h-BN Nanosheeets with High Photocatalytic Activity. Adv. Mater. Interfaces 2019, 6, 1900062.
- Sun, M.; Lv, Y.; Song, Y.; Wu, H.; Wang, G.; Zhang, H.; Chen, M.; Fu, Q.; Bao, X. CO-Tolerant /C Core–Shell Electrocatalysts for Proton Exchange Membrane Fuel Cells. Appl. Surf. Sci. 2018, 450, 244–250.
- Yesudoss, D.K.; Lee, G.; Shanmugam, S. Strong Catalyst Support Interactions in Defect-Rich γ-Mo2N Nanoparticles Loaded 2D-h-BN Hybrid for Highly Selective Nitrogen Reduction Reaction. Appl. Catal. B-Environ. 2021, 287, 119952.
- Min, Y.; Zhou, X.; Chen, J.-J.; Chen, W.; Zhou, F.; Wang, Z.; Yang, J.; Xiong, C.; Wang, Y.; Li, F.; et al. Integrating Single-Cobalt-Site and Electric Field of Boron Nitride in Dechlorination Electrocatalysts by Bioinspired Design. Nat. Comm. 2021, 12, 303.
- Balta, Z.; Simsek, E.B. Understanding the Structural and Photocatalytic Effects of Incorporation of Hexagonal Boron Nitride Whiskers into Ferrite Type Perovskites (BiFeO3, MnFeO3) for Effective Removal of Pharmaceuticals from Real Wastewater. J. Alloys Compd. 2022, 898, 162897.
- Merlo, A.; Mokkapati, V.R.S.S.; Pandit, S.; Mijakovic, I. Boron Nitride Nanomaterials: Biocompatibility and Bio-applications. Biomater. Sci. 2018, 6, 2298–2311.
- Sen, O.; Melis, E.; Çulha, M. One-Step Synthesis of Hexagonal Boron Nitrides, Their Crystallinity and Biodegradation. Front. Bioeng. Biotechnol. 2018, 6, 83.
- Şen, Ö.; Emanet, M.; Çulha, M. Stimulatory Effect of Hexagonal Boron Nitrides in Wound Healing. ACS Appl. Bio Mater. 2019, 2, 5582–5596.
- Sogut, I.; Paltun, S.O.; Tuncdemir, M.; Ersoz, M.; Hurdag, C. The Antioxidant and Antiapoptotic Effect of Boric Acid on Hepatoxicity in Chronic Alcohol-Fed Rats. Can. J. Physiol. Pharmacol. 2018, 96, 404–411.
- Donbaloglu, F.; Leblebici, S. Interactive Effect of Boric Acid and Temperature Stress on Phenological Characteristics and Antioxidant System in Helianthus annuus L. South Afr. J. Bot. 2022, 147, 391–399.
- Ince, S.; Kucukkurt, I.; Cigerci, H.I.; Fidan, A.F.; Eryavuz, A. The Effects of Dietary Boric Acid and Borax Supplementation on Lipid Peroxidation, Antioxidant Activity, and DNA Damage in Rats. J. Trace Elem. Med. Biol. 2010, 24, 161–164.
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent Developments in the Medicinal Chemistry of Single Boron Atom-Containing Compounds. Acta Pharm. Sin. B 2021, 11, 3035–3059.
- Taskin, I.C.; Sen, O.; Emanet, M.; Culha, M. Hexagonal Boron Nitrides Reduce the Oxidative Stress on Cells. Nanotechnology 2020, 31, 215101.
- Kar, F.; Söğüt, I.; Hacioğlu, C. Hexagonal Boron Nitride Nanoparticles Trigger Oxidative Stress by Modulating Thiol/Disulfide Homeostasis. Hum. Exp. Toxicol. 2021, 40, 1572–1583.
- Yegin, B.; Ozkazanc, H.; Er, K.D.; Ozkazanc, E. Antimicrobial Performance and Charge Transport Mechanism of Poly(N-Methylpyrrole)-Boron Nitride Composite. Mater. Chem. Phys. 2022, 278, 125709.
- Pandit, S.; Gaska, K.; Mokkapati, V.R.S.S.; Forsberg, S.; Svensson, M.; Kádár, R.; Mijakovic, I. Antibacterial effect of boron nitride flakes with controlled orientation in polymer composites. RSC Adv. 2019, 9, 33454–33459.
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Sarih, N.M.; Sudesh, K.; Khan, N.A.; Saidur, R.; Sridewi, N. Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications. Nanomaterials 2019, 9, 645.
- Xiong, S.; Fu, P.; Zou, Q.; Chen, L.; Jiang, M.; Zhang, P.; Wang, Z.; Cui, L.; Guo, H.; Gai, J. Heat Conduction and Antibacterial Hexagonal Boron Nitride/Polypropylene Nanocomposite Fibrous Membranes for Face Masks with Long-Time Wearing Performance. ACS Appl. Mater. Interfaces 2020, 13, 196–206.
- Onyszko, M.; Markowska-Szczupak, A.; Rakoczy, R.; Paszkiewicz, O.; Janusz, J.; Gordon-Kuza, A.; Wenelska, K.; Mijowska, E. Few Layered Oxidized h-BN as Nanofiller of Cellulose-Based Paper with Superior Antibacterial Response and Enhanced Mechanical/Thermal Performance. Int. J. Mol. Sci. 2020, 21, 5396.
- Gudz, K.Y.; Antipina, L.Y.; Permyakova, E.S.; Kovalskii, A.M.; Konopatsky, A.S.; Filippovich, S.Y.; Dyatlov, I.A.; Slukin, P.V.; Ignatov, S.G.; Shtansky, D.V. Ag-Doped and Antibiotic-Loaded Hexagonal Boron Nitride Nanoparticles as Promising Carriers to Fight Different Pathogens. ACS Appl. Mater. Interfaces 2021, 13, 23452–23468.
- Kivanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of Hexagonal Boron Nitride Nanoparticles on Antimicrobial and Antibiofilm Activities, Cell Viability. Mater. Sci. Eng. C 2018, 91, 115–124.
- Sert, B.; Gonca, S.; Ozay, Y.; Harputlu, E.; Ozdemir, S.; Ocakoglu, K. Biointerfaces Investigation of the Antifouling Properties of Polyethersulfone Ultrafiltration Membranes by Blending of Boron Nitride Quantum Dots. Colloid Surf. B 2021, 205, 111867.
- Zhang, Y.; Chan, C.; Li, Z.; Ma, J.; Meng, Q.; Zhi, C.; Sun, H.; Fan, J. Nanotoxicity of Boron Nitride Nanosheet to Bacterial Membranes. Langmuir 2019, 35, 6179–6187.
- Gudz, K.Y.; Permyakova, E.S.; Matveev, A.T.; Bondarev, A.V.; Manakhov, A.M.; Sidorenko, D.A.; Filippovich, S.Y.; Brouchkov, A.V.; Golberg, D.V.; Ignatov, S.G.; et al. Pristine and Antibiotic-Loaded Nanosheets/Nanoneedles-Based Boron Nitride Films as a Promising Platform to Suppress Bacterial and Fungal Infections. ACS Appl. Mater. Interfaces 2020, 12, 42485–42498.
- Rasel, M.A.I.; Li, T.; Nguyen, T.D.; Singh, S.; Zhou, Y.; Gu, Y.T. Biophysical Response of Living Cells to Boron Nitride Nanoparticles: Uptake Mechanism and Bio-Mechanical Characterization. J. Nanoparticle Res. 2015, 17, 441.
- Li, X.; Wang, X.; Zhang, J.; Hanagata, N.; Wang, X.; Weng, Q.; Ito, A.; Bando, Y.; Golberg, D. Hollow Boron Nitride Nanospheres as Boron Reservoir for Prostate Cancer Treatment. Nat. Commun. 2017, 8, 13936.
- Volkmann, M.; Meyns, M.; Lesyuk, R.; Lehmann, H.; Klinke, C. Attachment of Colloidal Nanoparticles to Boron Nitride Nanotubes. Chem. Mater. 2017, 29, 726–734.
- Ikram, M.; Hussain, I.; Hassan, J.; Haider, A.; Imran, M.; Aqeel, M.; Ul-hamid, A.; Ali, S. Evaluation of Antibacterial and Catalytic Potential of Copper-Doped Chemically Exfoliated Boron Nitride Nanosheets. Ceram. Int. 2020, 46, 21073–21083.
- Ikram, M.; Jahan, I.; Haider, A.; Hassan, J.; Ul-Hamid, A.; Imran, M.; Haider, J.; Shahzadi, A.; Shahbaz, A.; Ali, S. Bactericidal Behavior of Chemically Exfoliated Boron Nitride Nanosheets Doped with Zirconium. Appl. Nanosci. 2020, 10, 2339–2349.
- Pan, Y.; Zheng, H.; Li, G.; Li, Y.; Jiang, J.; Chen, J.; Xie, Q.; Wu, D.; Ma, R.; Liu, X.; et al. Antibiotic-Like Activity of Atomic Layer Boron Nitride for Combating Resistant Bacteria. ACS Nano 2022, 16, 7674–7688.
- Xie, X.; Hou, Z.; Duan, G.; Zhang, S.; Zhou, H.; Yang, Z.; Zhou, R. Boron Nitride Nanosheets Elicit Significant Hemolytic Activity via Destruction of Red Blood Cell Membranes. Colloid Surf. B 2021, 203, 111765.
- Mapleback, B.J.; Brack, N.; Thomson, L.; Spencer, M.J.S.; Osborne, D.A.; Doshi, S.; Thostenson, E.T.; Rider, A.N. Development of Stable Boron Nitride Nanotube and Hexagonal Boron Nitride Dispersions for Electrophoretic Deposition. Langmuir 2020, 36, 3425–3438.
- Czarniewska, E.; Mrówczyńska, L.; Jędrzejczak-Silicka, M.; Nowicki, P.; Trukawka, M.; Mijowska, E. Non-Cytotoxic Hydroxyl-Functionalized Exfoliated Boron Nitride Nanoflakes Impair the Immunological Function of Insect Haemocytes in Vivo. Sci. Rep. 2019, 9, 14027.
- Maharjan, S.; Gautam, M.; Poudel, K.; Yong, C.S.; Ku, S.K.; Kim, J.O.; Byeon, J.H. Biomaterials Streamlined Plug-in Aerosol Prototype for Reconfigurable Manufacture of Nano-Drug Delivery Systems. Biomaterials 2022, 284, 121511.
- Pasquale, D.D.; Marino, A.; Tapeinos, C.; Pucci, C. Homotypic Targeting and Drug Delivery in Glioblastoma Cells Through Cell Membrane-Coated Boron Nitride Nanotubes. Mater. Des. 2020, 192, 108742.
- Liu, J.; Zheng, T.; Tian, Y. Photodynamic Therapy Functionalized h-BN Nanosheets as a Theranostic Platform for SERS Real-Time Monitoring of MicroRNA and Photodynamic Therapy Angewandte. Angew. Chem. Int. Ed. 2019, 58, 7757–7761.
- Ahmad, P.; Khandaker, M.U.; Muhammad, N.; Rehman, F.; Ullah, Z.; Khan, G.; Khan, M.I.; Haq, S.; Ali, H.; Khan, A.; et al. Synthesis of Enriched Boron Nitride Nanocrystals: A Potential Element for Biomedical Applications. Appl. Radiat. Isot. 2020, 166, 109404.
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820.
- Yokoyama, K.; Miyatake, S.-I.; Kajimoto, Y.; Kawabata, S.; Doi, A.; Yoshida, T.; Asano, T.; Kirihata, M.; Ono, K.; Kuroiwa, T. Pharmacokinetic Study of BSH and BPA in Simultaneous Use for BNCT. J. Neurooncol. 2006, 78, 227–232.
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852.
- Li, L.; Dai, K.; Li, J.; Shi, Y.; Zhang, Z.; Liu, T.; Xie, J.; Zhang, R.; Liu, Z. A Boron-10 Nitride Nanosheet for Combinational Boron Neutron Capture Therapy and Chemotherapy of Tumor. Biomaterials 2021, 268, 120587.
- Silva, W.M.; Ribeiro, H.; Taha-Tijerina, J.J. Potential Production of Theranostic Boron Nitride Nanotubes (64 Cu-BNNTs) Radiolabeled by Neutron Capture. Nanomaterials 2021, 11, 2907.
- Shuai, C.; Gao, C.; Feng, P.; Xiao, T.; Yu, K.; Deng, Y.; Peng, S. Boron Nitride Nanotubes Reinforce Tricalcium Phosphate Scaffolds and Promote the Osteogenic Differentiation of Mesenchymal Stem Cells. J. Biomed. Nanotechnol. 2016, 12, 934–947.
- Li, R.; Lin, J.; Fang, Y.; Yu, C.; Zhang, J.; Xue, Y.; Liu, Z.; Zhang, J.; Tang, C.; Huang, Y. Porous Boron Nitride Nanofibers/PVA Hydrogels with Improved Mechanical Property and Thermal Stability. Ceram. Int. 2018, 44, 22439–22444.
- Belaid, H.; Nagarajan, S.; Barou, C.; Huon, V.; Bares, J.; Balme, S.; Miele, P.; Cornu, D.; Cavailles, V.; Teyssier, C.; et al. Boron Nitride Based Nanobiocomposites: Design by 3D Printing for Bone Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 1865–1874.
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yilmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D Printing of PVA/Hexagonal Boron Nitride/Bacterial Cellulose Composite Scaffolds for Bone Tissue Engineering. Mater. Des. 2020, 196, 109094.
- Özmeriç, A.; Tanoğlu, O.; Ocak, M.; Çelik, H.H.; Fırat, A.; Kaymaz, F.F.; Koca, G.; Şenes, M.; Alemdaroğlu, K.B.; Iltar, S.; et al. Intramedullary Implants Coated with Cubic Boron Nitride Enhance Bone Fracture Healing in a Rat Model. J. Trace Elem. Med. Biol. 2020, 62, 126599.
- Baghdadi, I.; Zaazou, A.; Tarboush, A.B.; Zakhour, M.; Özcan, M.; Salameh, Z. Physiochemical Properties of a Bioceramic-Based Root Canal Sealer Reinforced with Multi-Walled Carbon Nanotubes, Titanium Carbide and Boron Nitride Biomaterials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103892.
