Marine bioactive peptides (MBAPs) that are present in many marine species, including fish, sponges, cyanobacteria, fungi, ascidians, seaweeds, and mollusks, have gained widespread attention for their health-promoting benefits. MBAPs obtained from marine species have ameliorating potential against many health conditions, such as hypertension, diabetes, obesity, HIV, cancer, oxidation, and inflammation. Various research studies have indicated that MBAPs can be utilized as novel lead structures for the treatment of HIV in conjunction with pharmaceuticals and functional foods owing to their potential therapeutic, and antiretroviral (ARV) activities.
- bioactive peptides
- anti-HIV
- drugs
- marine organisms
- antiretroviral agents
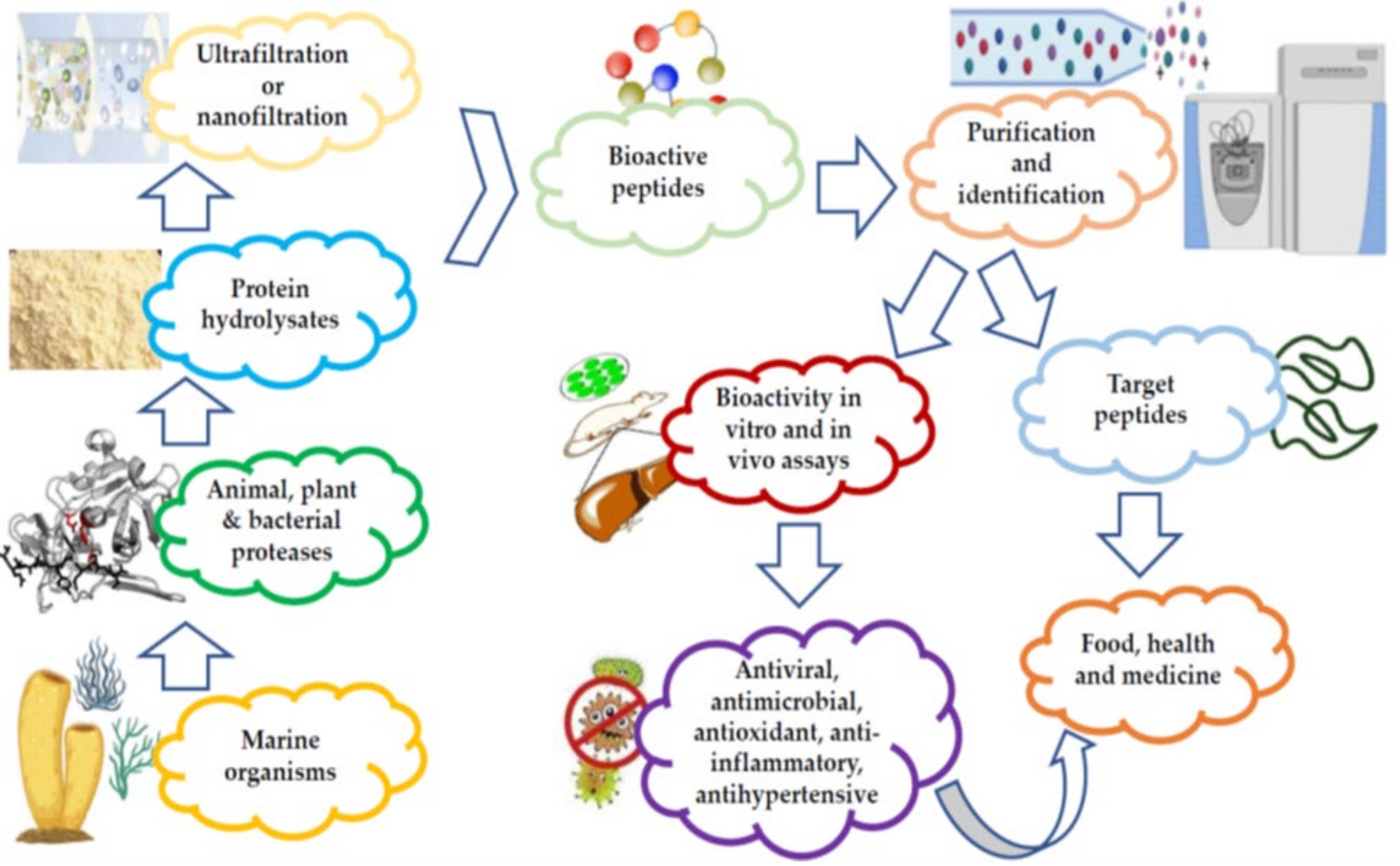
1. Techniques Used for the Commercial Preparation and Purification of Marine Bioactive Peptides
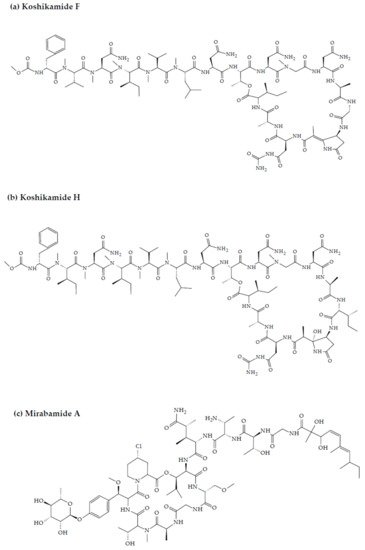
2. Marine Sponge-Derived Bioactive Peptides against HIV
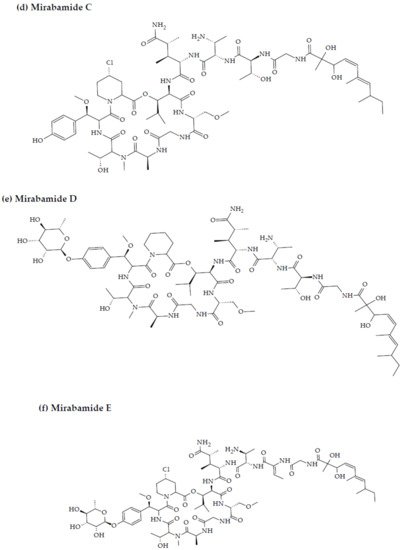
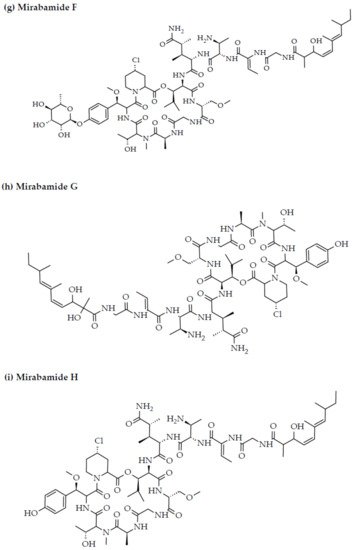
3. Marine Cyanobacteria-Associated Compounds with Anti-HIV Properties

This entry is adapted from the peer-reviewed paper 10.3390/md20080477
References
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228.
- Chakrabarti, S.; Guha, S.; Majumder, K. Food derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738.
- Martínez-Núñez, M.A.; López, V.E.L. Nonribosomal peptides synthetases and their applications in industry. Sustain. Chem. Process. 2016, 4, 13.
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, preparation and purification of marine bioactive peptides. Biomed Res. Int. 2017, 2017, 9746720.
- Le Gouic, A.; Harnedy, P.; Fitz Gerald, R. Bioactive peptides from fish protein byproducts. In Bioactive Molecules in Food, 1st ed.; Jean-Michel, M., Ramawat, K.G., Eds.; Springer: New York, NY, USA, 2018; pp. 1–35.
- Atef, M.; Chait, Y.A.; Ojagh, S.M.; Latifi, A.M.; Esmaeili, M.; Hammami, R.; Udenigwe, C.C. Anti-Salmonella activity and peptidomics profiling of peptide fractions produced from sturgeon fish skin collagen (Huso huso) using commercial enzymes. Nutrients 2021, 13, 2657.
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal. 2002, 13, 105–113.
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230.
- Ersson, B.; Rydén, L.; Janson, J.C. Protein Purification: Principles, High Resolution Methods, and Applications, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 1–22.
- Lee, J.W.; Jeffries, T.W. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour. Technol. 2011, 102, 5884–5890.
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T. Membrane Filtration in Advanced Physicochemical Treatment Processes; Humana Press: Totowa, NJ, USA, 2006; pp. 203–259.
- Bai, C.; Wei, Q.; Ren, X. Selective extraction of collagen peptides with high purity from cod skins by deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 7220–7227.
- Anbuchezian, R.; Ravichandran, S.; Karthick Rajan, D.; Tilivi, S.; Prabha-Devi, S. Identification and functional characterization of antimicrobial peptide from the marine crab Dromia dehaani. Microb. Pathog. 2018, 125, 60–65.
- Anand, M.; Alagar, M.; Ranjitha, J.; Selvaraj, V. Total synthesis and anticancer activity of a cyclic heptapeptide from marine sponge using water soluble peptide coupling agent EDC. Arab. J. Chem. 2019, 12, 2782–2787.
- Auwal, S.M.; Zainal Abidin, N.; Zarei, M.; Tan, C.P.; Saari, N. Identification, structure activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE 2019, 14, e0197644.
- Fan, X.; Cui, Y.; Zhang, R.; Zhang, X. Purification and identification of anti-obesity peptides derived from Spirulina platensis. J. Funct. Foods. 2018, 47, 350–360.
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Int. Food Res. 2017, 100, 416–422.
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and identification of antioxidant peptide from underutilized Dunaliella salina protein: Extraction, in vitro gastrointestinal digestion, and fractionation. Biomed Res. Int. 2019, 2019, 6424651.
- Xu, B.; Ye, L.; Tang, Y.; Zheng, J.; Tian, X.; Yang, Y.; Yang, Z. Preparation and purification of an immunoregulatory peptide from Stolephorus chinensis of the East Sea of China. Process Biochem. 2020, 98, 151–159.
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Int. Food Res. 2021, 147, 110468.
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S. Marine sponges as a drug treasure. Biomol. Ther. 2016, 24, 347–362.
- Vitali, A. Antimicrobial peptides derived from marine sponges. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1006.
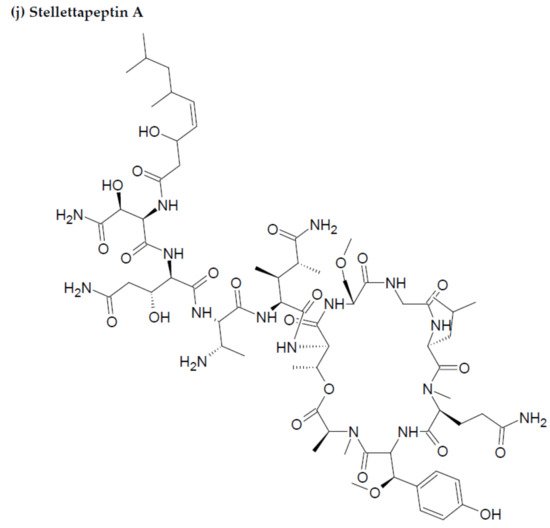
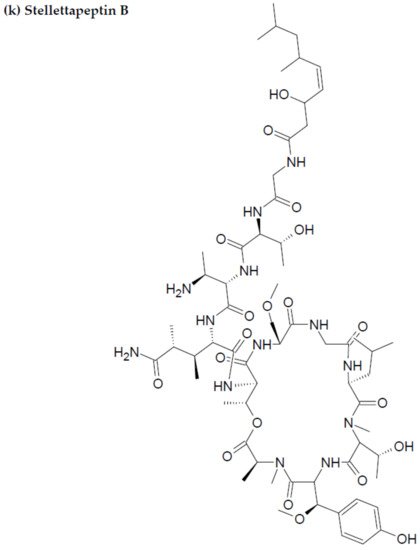
- Wu, Q.; Nay, B.; Yang, M.; Ni, Y.; Wang, H.; Yao, L.; Li, X. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharm. Sin. B. 2019, 9, 237–257.
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654.
- Plaza, A.; Bifulco, G.; Masullo, M.; Lloyd, J.R.; Keffer, J.L.; Colin, P.L.; Hooper, J.N.; Bell, L.J.; Bewley, C.A. Mutremdamide A and koshikamides C-H, peptide inhibitors of HIV-1 entry from different Theonella species. J. Org. Chem. 2010, 75, 4344–4355.
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333.
- Kang, H.; Choi, M.; Seo, C.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919.
- Macedo, M.W.F.S.; Cunha, N.B.; Carneiro, J.A.; Costa, R.A.; Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci 2021, 8, 889.
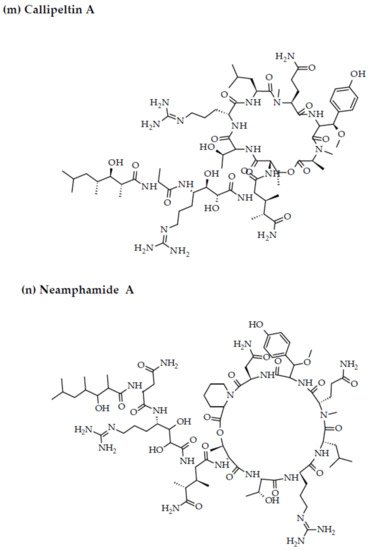
- Oku, N.; Gustafson, K.R.; Cartner, L.K.; Wilson, J.A.; Shigematsu, N.; Hess, S.; Pannell, L.K.; Boyd, M.R.; McMahon, J.B. Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod. 2004, 67, 1407–1411.
- Andavan, G.S.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834.
- Vo, T.; Kim, S. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892.
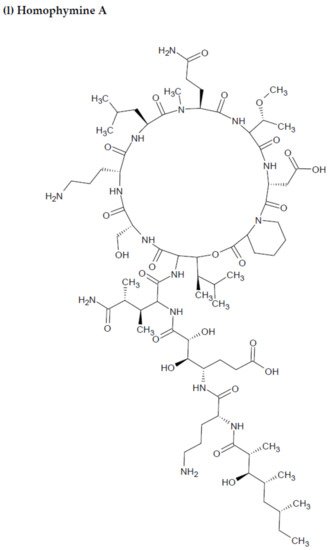
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P.; D’Auria, M.; Cresteil, T. Homophymines B–E and A1–E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044.
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Computational pharmacokinetics Report, ADMET study and conceptual DFT-based estimation of the chemical reactivity properties of marine cyclopeptides. Chem. Open 2021, 10, 1142–1149.
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N. Biotechnological applications of bioactive peptides from marine sources. Adv. Microb. Physiol. 2018, 73, 171–220.
- Sukmarini, L. Antiviral peptides (AVPs) of marine origin as propitious therapeutic drug candidates for the treatment of human viruses. Molecules 2022, 27, 2619.
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219.
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397.
- Chun, T.W.; Moir, S.; Fauci, A.S. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat. Immunol. 2015, 16, 584–589.
- Wang, M.; Tietjen, I.; Chen, M.; Williams, D.E.; Daoust, J.; Brockman, M.A.; Andersen, R.J. Sesterterpenoids isolated from the sponge Phorbas sp. activate latent HIV-1 provirus expression. J. Org. Chem. 2016, 81, 11324–11334.
- Maina, E.K.; Adan, A.A.; Mureithi, H.; Muriuki, J.; Lwembe, R.M. A review of current strategies towards the elimination of latent HIV-1 and subsequent HIV-1 cure. Curr. HIV Res. 2021, 19, 14–26.
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-and-lock strategies to cure HIV infection. Viruses 2020, 12, 84.
- Richard, K.; Williams, D.E.; De Silva, E.D.; Brockman, M.A.; Brumme, Z.L.; Andersen, R.J.; Tietjen, I. Identification of novel HIV-1 latency-reversing agents from a library of marine natural products. Viruses 2018, 10, 348.
- Silipo, A.; Molinaro, A.; Molteni, M.; Rossetti, C.; Parrilli, M.; Lanzetta, R. Full structural characterization of an extracellular polysaccharide produced by the freshwater cyanobacterium Oscillatoria planktothrix. Eur. J. Org. Chem. 2010, 2010, 5594–5600.
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: A review. Mar. Drugs 2019, 17, 561.
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530.
- Mazalovska, M.; Kouokam, J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. Biomed Res. Int. 2018, 2018, 3750646.
- Agarwal, R.; Trivedi, J.; Mitra, D. High yield production of recombinant cyanovirin-N (antiviral lectin) exhibiting significant anti-HIV activity, from a rationally selected Escherichia coli strain. Process Biochem. 2020, 93, 1–11.
- Lotfi, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. Bioimpacts 2018, 8, 139–151.
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da-Silva, D.M.; Cannon, P.M.; Kast, W.M. Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: Implications for prevention and treatment. AIDS Patient Care STDS 2016, 30, 291–306.
- Vamvaka, E.; Evans, A.; Ramessar, K.; Krumpe, L.R.; Shattock, R.J.; Keefe, B.R.; Christou, P.; Capell, T. Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Rep. 2016, 35, 1309–1319.
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel α (1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854.
- Shahid, M.; Qadir, A.; Yang, J.; Ahmad, I.; Zahid, H.; Mirza, S.; Windisch, M.P.; Shahzad-ul-Hussan, S. An engineered microvirin variant with identical structural domains potently inhibits human immunodeficiency virus and hepatitis C virus cellular entry. Viruses 2020, 12, 199.
- Armario-Najera, V.; Blanco-Perera, A.; Shenoy, S.R.; Sun, Y.; Marfil, S.; Muñoz-Basagoiti, J.; Perez-Zsolt, D.; Blanco, J.; Izquierdo-Useros, N.; Capell, T.; et al. Physicochemical characterization of the recombinant lectin scytovirin and microbicidal activity of the SD1 domain produced in rice against HIV-1. Plant Cell Rep. 2022, 41, 1013–1023.
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivi. Res. 2014, 112, 1–7.
- Jones, T.H.; McClelland, E.E.; McFeeters, H.; McFeeters, R.L. Novel antifungal activity for the lectin scytovirin: Inhibition of Cryptococcus neoformans and Cryptococcus gattii. Front. Microbiol. 2017, 8, 755.
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497.
- Xiong, C.; O’Keefe, B.R.; Botos, I.; Wlodawer, A.; McMahon, J.B. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 2006, 46, 233–239.
