Nickel-Copper (Ni-Cu) alloys exhibit simultaneously high strength and toughness (particularly, at cryogenic temperatures), excellent corrosion resistance, and may show good wear resistance. Therefore, they are widely used for manufacturing of (i) structural components of equipment in the chemical, oil, and marine industries, (ii) resistors and contacts in electrical and electronic equipment, (iii) corrosion resistant coatings, and (iv) fuel cells. Processing technologies includes bar forging, plate and tube rolling, wire drawing, heat treatment (for certain alloy compositions), powder and wire arc additive manufacturing, electrodeposition.
- Nickel-Copper alloys
- cryogenic equipment
- corrosion protection
1. Introduction
The Ni-Cu system forms the basis for the Monel alloy family (Table 1). Monel was discovered by Robert Crooks Stanley who was employed by the International Nickel Company (INCO) in 1901. The new alloy was named in honour of the company president, Ambrose Monell. The name is now a trademark of Special Metals Corporation [1].
Table 1. Chemical compositions of Monel alloys (wt.%).
| Monel Alloys | Ni | Cu | C | Mn | Fe | Co | S | Si | Al | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monel 400 | Max | - | 34.0 | 0.3 | 2.0 | 2.5 | - | 0.024 | 0.5 | - | - |
| Min | 63.0 | 28.0 | - | - | - | - | - | - | - | - | |
| Monel 401 | Max | 45.0 | Balance | 0.1 | 2.25 | 0.75 | 0.25 | 0.015 | 0.25 | - | - |
| Min | 40.0 | - | - | - | - | - | - | - | - | - | |
| Monel 404 | Max | 57.0 | Balance | 0.15 | 0.1 | 0.5 | - | 0.024 | 0.1 | 0.05 | - |
| Min | 52.0 | - | - | - | - | - | - | - | - | - | |
| Monel R405 | Max | - | 34.0 | 0.3 | 2.0 | 2.5 | - | 0.060 | 0.5 | - | - |
| Min | 63.0 | 28.0 | - | - | - | - | 0.025 | - | - | - | |
| Monel K500 | Max | - | 33.0 | 0.18 | 1.5 | 2.0 | 0.25 | 0.006 | 0.5 | 3.15 | 0.85 |
| Min | 63.0 | 27.0 | - | - | - | - | - | - | 2.3 | 0.35 |
Ni and Cu exhibit very similar atomic characteristics. They both have face centred cubic (fcc) crystal structure type, less than three percent difference in atomic radii, and exhibit similar electronegativity and valence state. The Ni-Cu system has complete solid solubility, which allows production of single phase alloys over the entire composition range [2]. Although the Ni-Cu system exhibits complete solid solubility [3], the large differences in melting points between Ni (1455 °C) and Cu (1085 °C) can result in Cu segregation. Following equilibrium solidification at slow cooling rates, dendrites become enriched in Ni and interdendritic regions get enriched in Cu [4,5,6]. However, with an increase in cooling rate during solidification the compositional gradient decreases and the microstructure morphology changes from dendritic to cellular [7]. Higher undercoolings during solidification also lead to finer and more equiaxed grain sizes after annealing [8].
Monel alloys can be easily fabricated by hot and cold metal forming processes and machining. Recrystallisation studies determined the optimum hot deformation temperatures to be 950–1150 °C [9,10], which are quite similar to other Ni-base alloys and steels. However, heat treatment schedule requires rigorous development: usually a two-step age-hardening heat treatment in the temperature range of 650–480 °C is used for Ni-Cu alloys [11,12]. The higher temperature stage helps to quickly nucleate precipitates of alloying elements, and the lower temperature stage provides a superior distribution of higher number density of smaller-sized particles. Ni-Cu alloys are usually quite weldable to each other and to other Ni alloys and stainless steels [13,14]. Lower heat inputs produce finer grain microstructures with random texture and higher strength and ductility [15]. Monel alloys are expensive, with their cost reaching up to 3 times that of Ni and 7 times that of Cu [16,17,18]. Hence their use is limited to those applications where they cannot be replaced with a cheaper alternative.
Major additions of copper (28–40 wt.%) improve corrosion resistance of Ni in many agents, in particular nonoxidizing acids, nonaerated sulphuric and hydrofluoric acids [19,20,21]. This determines areas of application of Ni-Cu alloys. They are widely used for manufacturing various components of equipment in chemical, oil and marine industries (such as drill collars, pumps, valves, fixtures, piping, fasteners, screws, propeller shafts, steam generators, turbines [22,23,24,25,26,27]), for protective coating [28,29], for manufacturing electrical and electronic equipment (resistors, bimetal contacts, capsules for transistors and ceramic-to-metal sealing [30,31]), and in fuel cells [32,33].
2. Mechanical Properties
Room temperature tensile properties of Monel 400 and K500 are shown in Table 2 [20]. In hot-rolled and annealed conditions Monel K500 shows a 100–200 MPa higher yield stress (YS) and ultimate tensile strength (UTS) than Monel 400, due to solid solution and precipitation strengthening. In both alloys YS and UTS are slightly higher for cold formed products, due to work hardening. In hot finished Monel K500, annealing may lead to strength decrease by about 30%, following dissolution of precipitates and dislocation annihilation. In contrast, age-hardening may result in 1.3–2.5 times increase in strength due to precipitation. For the cold formed products, annealing decreases strength by about 40–50%; although, age-hardening may increase strength by up to 1.3 times. As seen, the age-hardening heat treatment is more effective in increasing strength of the hot finished products, compared to the cold finished.
Table 2. Mechanical properties of Monel 400 and K500 [20].
| Processing Condition | Yield Strength, MPa | Tensile Strength, MPa | Elongation, % | HB |
|---|---|---|---|---|
| Monel 400 | ||||
| Hot-Finished | 280–690 | 550–760 | 30–60 | 140–240 |
| Hot-Finished, Annealed | 170–340 | 520–620 | 35–60 | 110–150 |
| Cold-Drawn | 380–690 | 580–830 | 22–40 | 160–225 |
| Monel K500 | ||||
| Hot-Finished | 280–760 | 620–1070 | 45–20 | 140–315 |
| Hot-Finished, Aged | 690–1034 | 965–1310 | 30–20 | 265–346 |
| Hot-Finished, Annealed | 280–414 | 621–760 | 45–25 | 140–185 |
| Hot-Finished, Annealed and Aged | 586–830 | 896–1140 | 35–20 | 250–315 |
| Cold-Drawn, As-Drawn | 483–860 | 690–965 | 35–13 | 175–260 |
| Cold-Drawn, Aged | 655–1100 | 931–1280 | 30–15 | 255–370 |
| Cold-Drawn, Annealed | 280–414 | 621–760 | 50–25 | 140–185 |
| Cold-Drawn, Annealed and Aged | 586–830 | 896–1310 | 30–20 | 250–315 |
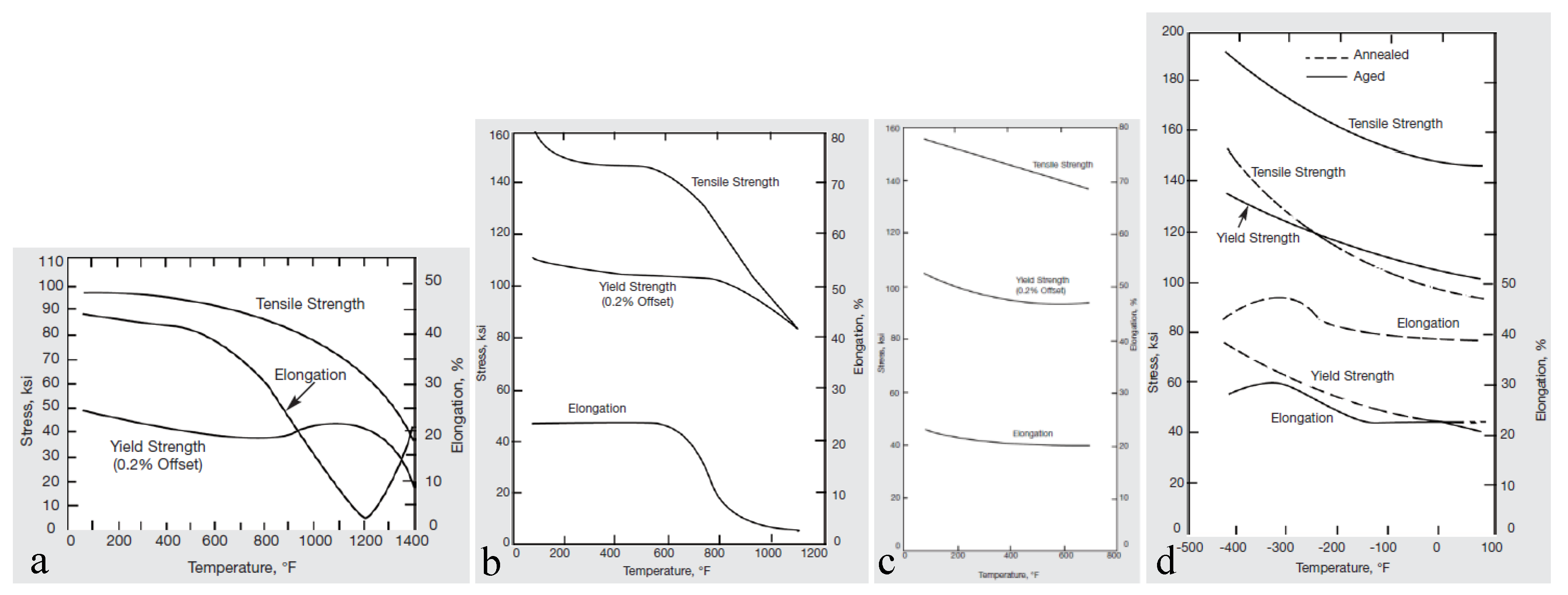
High and low temperature tensile properties of Monel K500 are shown in Figure 1. For hot rolled product, with an increase in temperature the YS and UTS do not vary significantly until 650 °C (1200 °F) and 150 °C (300 °F), respectively. Age-hardening increases YS and UTS by about 1.5 and 2 times, respectively. However, the YS stability at high temperature decreases, YS starts going down at a lower temperature of 430 °C (800 °F) compared to the non-aged material. In contrast, the stability of UTS increases, UTS starts decreasing at a higher temperature of 300 °C (550 °F) compared to the non-aged material. The decrease in YS stability after ageing can be associated with destruction of the dislocation sub-structure during ageing, and the increase in UTS stability follows particle precipitation. Annealing prior age-hardening does not provide any strength gain compared to the hot-rolled and age-hardened product, however the stability of elongation increase. This is a consequence of significantly reduced dislocation density after annealing and increased distance of dislocations free pass. With a decrease in temperature, the tensile strength and yield stress both increase while ductility and toughness remain virtually the same (Figure 1d). No ductile-to-brittle transition occurs even at temperatures as low as that of liquid hydrogen. Thus, this alloy is suitable for many cryogenic applications.
In view of excellent corrosion resistance coupled with high strength and toughness at cryogenic temperatures [95,96] Monel alloys are good candidates for structural components of machinery and storage of liquefied gases in aerospace and chemical industry. High temperature application of Monel is limited by low melting temperature of Cu. However, the precipitation strengthening capacity in the Ni-Cu alloy system can be improved with appropriate alloying element additions and heat treatment. Ni-Cu alloys are easily weldable [97] and were successfully used as an input material in powder based [98] and wire arc additive manufacturing [99]. Development of these modern technologies allows to apply the Ni-Cu alloys as a surface cladding material for protection of less corrosion resistant core or in components with mechanical properties gradient.
3. Strengthening mechanisms
Due to Ni and Cu exhibiting complete solid solubility, their alloys are single phase. Thus, in Ni-Cu alloy four strengthening mechanisms operate: grain refinement, solid solution, precipitation, and dislocation strengthening (work hardening). Composition of an alloy determines whether the solid solution or precipitation strengthening dominates. Mo, Ti, Cr, and Mn are the most frequently used solid solution strengthening elements, due to their significant difference in atomic sizes from Ni. Such elements as Fe, Co, and Cu are the second order solid solution strengtheners, due to their high solubility in Ni. Al and Ti are the most effective precipitation strengthening elements in Ni-Cu alloys, as they tend to form NiAlTi-rich intermetallic particles. Sometimes Mn-rich M23C6 or Ti-rich MC carbides can also precipitate. The dislocation strengthening capacity of Ni-Cu alloys is substantial, which is determined by their fcc crystal structure. However, cold deformation above 20% would decrease ductility below the practically reasonable limits of 30% elongation. In presence of particle forming elements in alloy composition, age hardening heat treatment is frequently used as the final strengthening operation.
This entry is adapted from the peer-reviewed paper 10.3390/met10101358
