The deregulation of apoptosis is involved in the development of several pathologies, and recent evidence suggests that apoptosis may be involved in chronic pain, namely in neuropathic pain. Neuropathic pain is a chronic pain state caused by primary damage or dysfunction of the nervous system. Recently, it was found that nerve endings contain transient receptor potential (TRP) channels that sense and detect signals released by injured tissues and respond to these damage signals. TRP channels are similar to the voltage-gated potassium channels or nucleotide-gated channels that participate in calcium and magnesium homeostasis. TRP channels allowing calcium to penetrate into nerve terminals can activate apoptosis, leading to nerve terminal destruction. Further, some TRPs are activated by acid and reactive oxygen species (ROS). ROS are mainly produced in the mitochondrial respiratory chain, and an increase in ROS production and/or a decrease in the antioxidant network may induce oxidative stress (OS). Depending on the OS levels, they can promote cellular proliferation and/or cell degeneration or death. Previous studies have indicated that proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), play an important role in the peripheral mediation of neuropathic pain.
- apoptosis
- pain
- cell signaling
- oxidative stress
- inflammation
1. Definition and Mechanisms of Pain
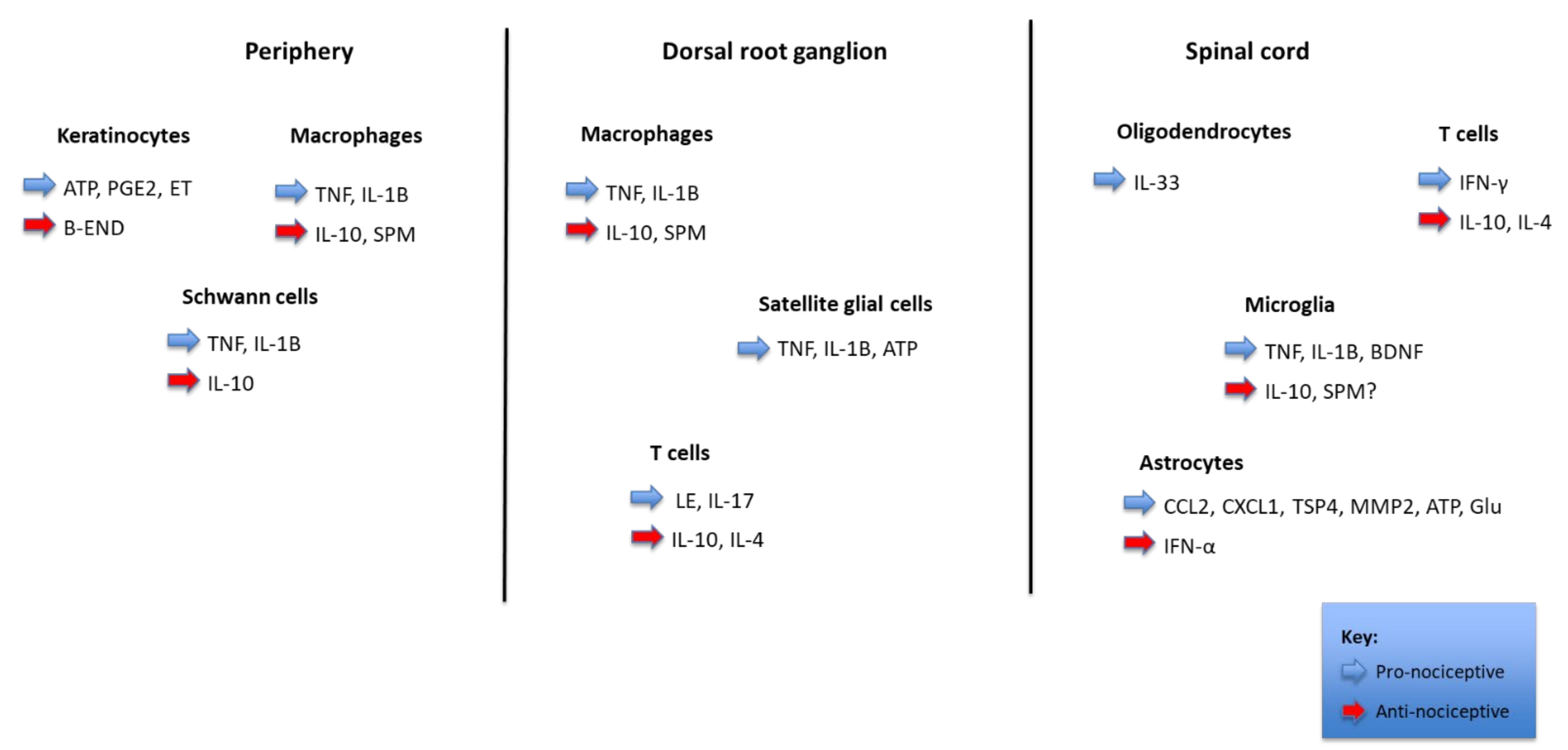
2. Apoptosis Pathways as Mediators of Pain Formation
2.1. Apoptosis Cell Signaling Pathways
2.2. Ubiquitin Proteasome Pathway and Apoptosis
2.3. Endoplasmic Reticulum Stress Signalling and Apoptosis
2.4. Oxidative Stress
2.5. Inflammation
2.6. The p38MAPK Pathway and Apoptosis
3. Apoptosis and Clinical Implications
3.1. Apoptosis and Neuropathic Pain
2.2. Biomarkers and Circulating Mediators in Pain
2.3. Pain Therapy through Modulating Apoptosis Activities
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10061255
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982.
- Nobelstiftelsen. Nobel Lectures in Physiology or Medicine; Elsevier for the Nobel Foundation: Amsterdam, The Netherlands; London, UK, 1965; pp. 1922–1941.
- Valenzuela-Moguillansky, C.; Reyes-Reyes, A.; Gaete, M.I. Exteroceptive and interoceptive body-self awareness in fibromyalgia patients. Front. Hum. Neurosci. 2017, 11, 117.
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577.
- Garcia-Larrea, L.; Peyron, R. Pain matrices and neuropathic pain matrices: A review. Pain 2013, 154, S29–S43.
- Orr, P.M.; Shank, B.C.; Black, A.C. The role of pain classification systems in pain management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418.
- Adams, J.D. Editorial: Apoptosis is critical to pain control. Open J. Apoptosis 2013, 2, 23–24.
- Ogawa, N.; Kurokawa, T.; Mori, Y. Sensing of redox status by TRP channels. Cell Calcium 2016, 60, 115–122.
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018, 9, 95.
- Nazıroğlu, M.; Braidy, N. Thermo-sensitive TRP channels: Novel targets for treating chemotherapy-induced peripheral pain. Front. Physiol. 2017, 8, 1040.
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in pain sensation. Front. Physiol. 2017, 8, 392.
- Yowtak, J.; Wang, J.; Kim, H.Y.; Lu, Y.; Chung, K.; Chung, J.M. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain 2013, 154, 2469–2476.
- Mai, L.; Huang, F.; Zhu, X.; He, H.; Fan, W. Role of nerve growth factor in orofacial pain. J. Pain Res. 2020, 13, 1875–1882.
- Braidy, N.; Smani, T.; Naziroglu, M. Editorial: Involvements of TRP channels, oxidative stress and apoptosis in neurodegenerative diseases. Front. Physiol. 2021, 12, 649230.
- Duitama, M.; Vargas-López, V.; Casas, Z.; Albarracin, S.L.; Sutachan, J.-J.; Torres, Y.P. TRP channels role in pain associated with neurodegenerative diseases. Front. Neurosci. 2020, 14, 782.
- Haraguchi, K.; Kawamoto, A.; Isami, K.; Maeda, S.; Kusano, A.; Asakura, K.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; Kaneko, S. TRPM2 Contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J. Neurosci. 2012, 32, 3931–3941.
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediat. Inflamm. 2017, 2017, 5048616.
- Suter, M.R.; Wen, Y.-R.; Decosterd, I.; Ji, R.-R. Do glial cells control pain? Neuron Glia Biol. 2007, 3, 255–268.
- Sui, B.; Xu, T.-Q.; Liu, J.-W.; Wei, W.; Zheng, C.-X.; Guo, B.-L.; Wang, Y.-Y.; Yang, Y.-L. Understanding the role of mitochondria in the pathogenesis of chronic pain. Postgrad. Med. J. 2013, 89, 709–714.
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295.
- Ding, R.; Sun, B.; Liu, Z.; Yao, X.; Wang, H.; Shen, X.; Jiang, H.; Chen, J. Advanced oxidative protein products cause pain hypersensitivity in rats by inducing dorsal root ganglion neurons apoptosis via NADPH Oxidase 4/c-Jun N-terminal kinase pathways. Front. Mol. Neurosci. 2017, 10, 195.
- Liao, M.-F.; Lu, K.-T.; Hsu, J.-L.; Lee, C.-H.; Cheng, M.-Y.; Ro, L.-S. The role of autophagy and apoptosis in neuropathic pain formation. Int. J. Mol. Sci. 2022, 23, 2685.
- Yuan, J.; Yankner, B.A. Apoptosis in the nervous system. Nature 2000, 407, 802–809.
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231.
- Duke, R.C.; Ojcius, D.M.; Young, J.D.-E. Cell suicide in health and disease. Sci. Am. 1996, 275, 80–87.
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992.
- Sekiguchi, M.; Sekiguchi, Y.; Konno, S.-I.; Kobayashi, H.; Homma, Y.; Kikuchi, S.-I. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur. Spine J. 2009, 18, 1978–1985.
- Corasaniti, M.T.; Amantea, D.; Russo, R.; Bagetta, G. The crucial role of neuronal plasticity in pain and cell death. Cell Death Differ. 2006, 13, 534–536.
- He, Q.; Wang, T.; Ni, H.; Liu, Q.; An, K.; Tao, J.; Chen, Y.; Xu, L.S.; Zhu, C.; Yao, M.; et al. Endoplasmic reticulum stress promoting caspase signaling pathway-dependent apoptosis contributes to bone cancer pain in the spinal dorsal horn. Mol. Pain 2019, 15, 1744806919876150.
- Peter, M.E.; Kischkel, F.C.; Hellbardt, S.; Chinnaiyan, A.M.; Krammer, P.H.; Dixit, V.M. CD95 (APO-1/Faz)—associating signalling proteins. Cell Death Differ. 1996, 3, 161–170.
- Lewin, G.R.; Nykjaer, A. Pro-neurotrophins, sortilin, and nociception. Eur. J. Neurosci. 2014, 39, 363–374.
- Deng, Y.; Yang, L.; Xie, Q.; Yang, F.; Li, G.; Zhang, G.; Li, S.; Wu, Z.; Wang, J.; Kang, X. Protein kinase a is involved in neuropathic pain by activating the p38MAPK pathway to mediate spinal cord cell apoptosis. Mediat. Inflamm. 2020, 2020, 6420425-17.
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916.
- Liu, X.-H.; Kwon, D.; Schielke, G.P.; Yang, G.-Y.; Silverstein, F.S.; Barks, J.D.E. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J. Cereb. Blood Flow Metab. 1999, 19, 1099–1108.
- Sarmento-Ribeiro, A.B.; Dourado, M.; Paiva, A.; Freitas, A.; Silva, T.; Regateiro, F.; Oliveira, C.R. Apoptosis deregulation influences chemoresistance to azaguanine in human leukemic cell lines. Cancer Investig. 2012, 30, 331–342.
- Li, B.; Dou, Q.P. Bax degradation by the ubiquitin/proteasome-dependent pathway: Involvement in tumor survival and progression. Proc. Natl. Acad. Sci. USA 2000, 97, 3850–3855.
- Cheng, J.; Deng, Y.; Zhou, J. Role of the ubiquitin system in chronic pain. Front. Mol. Neurosci. 2021, 14, 674914.
- Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. J. Biol. Chem. 2000, 275, 21648–21652.
- Zhang, H.-G.; Wang, J.; Yang, X.; Hsu, H.-C.; Mountz, J.D. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene 2004, 23, 2009–2015.
- Qiu, J.H.; Asai, A.; Chi, S.; Saito, N.; Hamada, H.; Kirino, T. Proteasome inhibitors induce cytochrome c–caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J. Neurosci. 2000, 20, 259–265.
- Hartung, J.E.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202.
- Niederberger, E.; Geisslinger, G. The IKK-NF-κB pathway: A source for novel molecular drug targets in pain therapy? FASEB J. 2008, 22, 3432–3442.
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563.
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665.
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45.
- Flatters, S.J. The contribution of mitochondria to sensory processing and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146.
- Bennett, G.J.; Doyle, T.; Salvemini, D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014, 10, 326–336.
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180.
- Lim, T.; Rone, M.B.; Lee, S.; Antel, J.P.; Zhang, J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol. Pain 2015, 11, 58.
- English, K.; Barton, M.C. HDAC6: A key link between mitochondria and development of peripheral neuropathy. Front. Mol. Neurosci. 2021, 14, 175.
- Todorova, V.; Blokland, A. Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr. Neuropharmacol. 2016, 15, 166–173.
- Haanen, C.; Vermes, I. Apoptosis and inflammation. Mediat. Inflamm. 1995, 4, 5–15.
- Wyllie, A.; Kerr, J.; Currie, A. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306.
- Wallach, D.; Kovalenko, A. Keeping inflammation at bay. eLife 2014, 3, e02583.
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257.
- Haslett, C.; Savill, J.S.; Whyte, M.K.B.; Stern, M.; Dransfield, I.; Meagher, L.C.; Nagata, S. Granulocyte apoptosis and the control of inflammation. Philos. Trans. R. Soc. B: Biol. Sci. 1994, 345, 327–333.
- Nagata, S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 2018, 36, 489–517.
- Tsuchiya, K. Switching from apoptosis to pyroptosis: Gasdermin-elicited inflammation and antitumor immunity. Int. J. Mol. Sci. 2021, 22, 426.
- Hart, S.P.; Dransfield, I.; Rossi, A.G. Phagocytosis of apoptotic cells. Methods 2008, 44, 280–285.
- Ellis, R.E.; Yuan, J.; Horvitz, H.R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 1991, 7, 663–698.
- Cohen, J.J. Apoptosis. Immunol. Today 1993, 14, 126–130.
- Arienti, S.; Barth, N.; Dorward, D.A.; Rossi, A.G.; Dransfield, I. Regulation of apoptotic cell clearance during resolution of inflammation. Front. Pharmacol. 2019, 10, 891.
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351.
- Martin, S.; Reutelingsperger, C.E.M.; McGahon, A.J.; Rader, J.; Van Schie, R.C.A.A.; LaFace, D.M.; Green, D. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556.
- Huynh, M.-L.N.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Investig. 2002, 109, 41–50.
- Segawa, K.; Nagata, S. an apoptotic ‘eat me’ signal: Phosphatidylserine exposure. Trends Cell Biol. 2015, 25, 639–650.
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974.
- Yamaguchi, H.; Maruyama, T.; Urade, Y.; Nagata, S. Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. eLife 2014, 3, e02172.
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, É.; Köröskényi, K. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Front. Immunol. 2017, 8, 909.
- Shin, J.; Yin, Y.; Park, H.; Park, S.; Triantafillu, U.L.; Kim, Y.; Kim, S.R.; Lee, S.Y.; Kim, D.K.; Hong, J.; et al. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine 2018, 13, 1607–1621.
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2010, 1802, 396–405.
- Zhu, G.-Q.; Liu, S.; He, D.-D.; Liu, Y.-P.; Song, X.-J. Activation of the cAMP-PKA signaling pathway in rat dorsal root ganglion and spinal cord contributes toward induction and maintenance of bone cancer pain. Behav. Pharmacol. 2014, 25, 267–276.
- Schoenberger, J.; Bauer, J.; Moosbauer, J.; Eilles, C.; Grimm, D. Innovative strategies in in vivo apoptosis imaging. Curr. Med. Chem. 2008, 15, 187–194.
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial protein that promotes cytochrome c–dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42.
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflügers Arch. Eur. J. Physiol. 2012, 463, 561–569.
- Kahya, M.C.; Nazıroğlu, M.; Övey, I.S. Modulation of diabetes-induced oxidative stress, apoptosis, and ca2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol. Neurobiol. 2016, 54, 2345–2360.
- Pi, Z.; Lin, H.; Yang, J. Isoflurane reduces pain and inhibits apoptosis of myocardial cells through the phosphoinositide 3-kinase/protein kinase B signaling pathway in mice during cardiac surgery. Mol. Med. Rep. 2018, 17, 6497–6505.
- Zhou, D.; Zhang, S.; Hu, L.; Gu, Y.-F.; Cai, Y.; Wu, D.; Liu, W.-T.; Jiang, C.-Y.; Kong, X.; Zhang, G.-Q. Inhibition of apoptosis signal-regulating kinase by paeoniflorin attenuates neuroinflammation and ameliorates neuropathic pain. J. Neuroinflammation 2019, 16, 83.
- Mei, X.-P.; Zhang, H.; Wang, W.; Wei, Y.-Y.; Zhai, M.-Z.; Wang, W.; Xu, L.-X.; Li, Y.-Q. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J. Neuroinflammation 2011, 8, 6.
- Tatsumi, E.; Yamanaka, H.; Kobayashi, K.; Yagi, H.; Sakagami, M.; Noguchi, K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia 2014, 63, 216–228.
- Leng, Y.-F.; Gao, X.-M.; Wang, S.-X.; Xing, Y.-H. Effects of tetramethylpyrazine on neuronal apoptosis in the superficial dorsal horn in a rat model of neuropathic pain. Am. J. Chin. Med. 2012, 40, 1229–1239.
- Ito, S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers 2020, 12, 265.
- Ahmed, A.; Ahmed, M.; Li, J.; Gu, H.F.; Bakalkin, G.; Stark, A.; Harris, H.E. Proteasome inhibitor MG132 modulates inflammatory pain by central mechanisms in adjuvant arthritis. Int. J. Rheum. Dis. 2014, 20, 25–32.
- Muscoli, C.; Dagostino, C.; Ilari, S.; Lauro, F.; Gliozzi, M.; Bardhi, E.; Palma, E.; Mollace, V.; Salvemini, D. Posttranslational nitration of tyrosine residues modulates glutamate transmission and contributes to N-Methyl-D-aspartate-Mediated thermal hyperalgesia. Mediat. Inflamm. 2013, 2013, 950947.
