Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The concept of the co-delivery approach first occurred from merging two research fields: drug delivery and gene therapy. Co-delivery systems combine at least two therapeutic agents with different physiological and physicochemical properties; thus, achieving clinical combination chemotherapy. Taking into consideration the co-delivery strategy, combination therapy via nanotechnology approaches has progressively become a desirable technique and one of the leading frontiers in research to find an efficient drug delivery system (DDS).
- gene
- co-delivery
- multi-drug resistance
- nanocarriers
- gene delivery
1. Introduction
Nanocarriers possess unique characteristics, such as a nanometric size, a high surface area to volume ratio, advantageous drug release patterns, and targeting qualities that can help them accumulate preferentially in tumor tissues [15]. Most importantly, nanomaterials can significantly increase the accumulation of their load at tumor locations by utilizing the enhanced permeability and retention effect (EPR) [4,30]. EPR is a phenomenon that is hypothesized to be caused by leaky tumor vasculature and poor lymphatic outflow. EPR is crucial in the realm of nanotechnology, specifically the passive targeting of nanoparticles in the tumor microenvironment, and it has been explored extensively by Galiardi et al. [13,25,27]. The development of more effective nanoparticles to deliver desired multiple payloads concurrently is a major challenge. Multiple biological parameters, such as interaction with plasma proteins, blood circulation time, extravasation, tumor tissue penetration, and cancer cell uptake, might alter the biodistribution of systemically administered nanoparticles [24]. Surface modification of nano-systems that bestow unique targeting capabilities or stimuli-sensitive responses impact the nanoparticles’ overall in vivo behavior [30,31]. Much of our present understanding of nanoparticle systems is based on in vitro studies and on animal models for in vivo data [25,30,32,33].
Various nanoparticles (NPs) have been examined to design novel co-delivery systems. There are two main groups of nanocarriers divided into inorganic-based and organic-based nanoparticles. Inorganic-based nanoparticles mostly include mesoporous silica nanoparticles, iron oxide nanoparticles, gold nanoparticles, quantum dots, etc.; whereas, organic-based nanoparticles include polymeric micelles, nano-emulsions, polymersomes, liposomes, dendrimers, noisomes, etc. [12,14,34] (Figure 1).
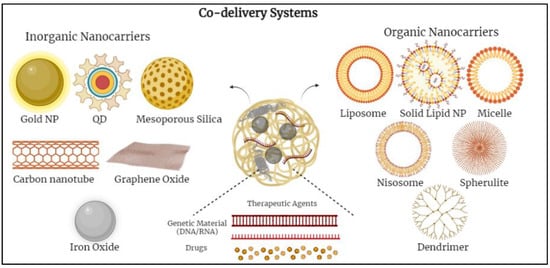
Figure 1. Schematic diagram demonstrating various co-delivery systems and the different inorganic- and organic-based nanocarriers.
2. Inorganic-Based Nanoparticles
Various inorganic nanoparticles have been developed, such as mesoporous silica nanoparticles (MSNs), gold nanoparticles (Au NPs), quantum dots, and iron-based nanoparticles. Using inorganic nanoparticles as co-delivery carriers frequently necessitates nanoparticle modification [4,13]. To accomplish an efficient and successful co-delivery, two or more physical or chemical modifications can be used. Even though inorganic nanoparticles have received a lot of interest as potential delivery vesicles, the FDA (Food and Drug Administration) and the EMA (European Medicines Agency) have only approved NanoThermVR as a drug delivery system (DDS) for limited anti-cancer therapy for glioblastoma, prostate, and pancreatic cancer [13,35]. None of the inorganic nanoparticles for co-delivery have been approved to be available on the market as they have not yet crossed the first clinical stages. Pegylated colloidal gold nanoparticles conjugated to tumor necrosis factor-alpha (TNFa) particles for cancer therapy and silicon nanocarriers for parenteral peptide administration, for example, are in early clinical development [4,32]. At the moment, gold and mesoporous silica nanoparticles are receiving the greatest attention in terms of experimentation and development [31].
2.1. Gold Nanoparticles (Au NPs)
Au NPs are considered an important breakthrough and have shown considerable potential in the delivery of chemotherapeutic agents for cancer treatment. The biocompatibility, adaptability, and non-immunogenic properties of Au NPs have allowed their incorporation into various treatments, such as radio-sensitizers, photothermal agents, and drug delivery vehicles [31]. Xiao et al. co-delivered the chemotherapeutic drug doxorubicin (DOX) and small interfering RNA (siRNA) against the achaete-scute complex-like 1 (ASCL1) gene, particularly to neuroendocrine cancer cells [35]. According to literature, ASCL1 is one of the most extensively expressed genes, and is involved in cell division and multiple other cellular functions, including intracellular transportation. ASCL1 protein expression in the body, and its role as a possible oncogene, are critical in the growth and advancement of several types and forms of cancer [36,37]. For targeting tumor cells specifically, the exterior surface of Au NPs can be altered by active targeting ligands or moieties. Au NPs passively accrue and retain preferentially in tumor locations because of the EPR effect [31,38]. Therefore, to also benefit from the acidic tumor microenvironment, a pH-sensitive multi-functional gold (Au) nanorod-based nanocarrier with distinctive properties was conjugated with octreotide (OCT) [4,32,36]. Octreotide is a cancer-targeting ligand, that attaches to overexpressed somatostatin receptors in neuroendocrine (NE) cancer cells [38]. The Au-DOX-OCT complexed with ASCL1 siRNA showed much stronger gene silencing and anti-proliferative effects in NE cancer cells than non-targeted Au-DOX complexed with ASCL1 siRNA [5,13,37]. This study reported synergistic effects between the co-delivered DOX and siRNA in inhibiting cell growth and cancer suppression. As a result, targeting cancer cells through a combination of chemotherapy and siRNA-mediated gene silencing using gold nanorods as nanocarriers has a lot of promise for improving therapeutic results in neuroendocrine tumors [32,36,39,40]. Other metal-based nanoparticles, such as iron oxide nanoparticles, have also been explored and possess several unique qualities because of their metal bonding, minute size effect, and surface properties [40]. Iron oxide nanoparticles that are minute and superparamagnetic with a diameter of roughly 10 nm can be utilized in thermotherapy and actively targeted cancer treatment [5,39]. It should be emphasized that when particle size decreases, some metal-based nanoparticles might interact with antioxidant defense mechanisms, resulting in increased cytotoxicity [18,41].
2.2. Mesoporous Silica Nanoparticles (MSNs)
Mesoporous silica nanoparticles (MSNs) are alternative key inorganic-based nanoparticles used in co-delivery. MSNs are optimal models for loading significant amounts of chemotherapeutic agents and pairing other components on the surface because of their high surface area to volume ratio and vast aperture volume [13,42,43]. The latter characteristics allow MSNs to be used in several cancer treatments. On the surface of MSNs, the chemical modification of a significant quantity of hydroxyl groups makes it easier for them to encapsulate medications or functionalize them later [35]. Meng et al. examined a co-delivery system that allocated the physical encapsulation of DOX molecules in the pores of MSNs, and microRNAs were conjugated onto the surface through disulfide bonds [5,15,43]. The experimental results depicted that, in vivo, the combination therapy exhibited improved therapeutic effectiveness and reduced systemic toxicity [28,43]. Several experimental studies showed some differences and variations between in vitro and in vivo outcomes. The MSNs induced endothelial injury and this damage appeared to coincide with thrombosis generated by MSN build-up and accrued in the tissues [43]. It is worth mentioning that by changing the nanoparticle surface, for example, by grafting poly(ethylene glycol), also known as PEGylation, the accumulation potential of silica nanoparticles may be considerably reduced [44]. Yiu et al. created magnetic core-encompassing MSNs covered with PEI (polyethyleneimine) for plasmid DNA (pDNA) transfer in vitro [45]. This nanoparticle design could also incorporate a targeting agent to offer selectivity in the tumor microenvironment where nucleic acids would get artificially introduced.
The PEI-coated MSN co-delivery system has gained significant acceptance among researchers; hence studies have already been conducted to analyze multiple drug delivery methods to enable synergistic therapeutic approaches [44,46,47]. Meng et al. created PEI-coated MSNs to transmit DOX and a siRNA that inhibits P-gp synthesis [47]. The study showed an increase in the cytotoxicity of the cancer cells combined with an MDR cell line using this dual delivery approach, compared to PEI-coated MSNs carrying only DOX [48,49]. Further studies showed that a refined methodology, delivering optimum proportions of P-gp –siRNA–DOX with PEGylated PEI-coated MSNs in an in vivo MDR breast cancer model, showed synergistic tumor growth reduction with considerable P-gp knockdown at heterogeneous tumor locations [50,51,52]. Wang et al. employed a technique quite similar to the technique Meng et al. used in treating squamous cell cancer (SCC) [47]. In this study, a significant reduction in tumor growth, ~80% decrease in 28 days, in vivo after the administration of PEI-coated MSNs to co-deliver DOX and siRNA against MDR1 (P-gp1) was observed [50,51,53].
2.3. Copper Nanoparticles (Cu NPs)
The continuous desire for novel therapeutic agents has fueled metal-based chemotherapeutic research. Metal-based therapeutic agents have generated the possibility of less toxic action against cancer cells and have demonstrated anti-proliferative action against cancer cells [53]. Copper has captured the attention of many researchers among metal-based nanoparticles due to its strong redox potential and its use as an adaptable carrier for drug delivery and phototherapy. Copper nanoparticles can change the regulation of over 1000 genes, such as metallothionein, glutathione synthetase, chaperones, histones, etc. [54,55]. Copper nanoparticles alter cell shape, reduce cell metabolic activity, raise oxidative stress, thereby causing mitochondrial damage, and eventually damage DNA by creating reactive oxygen species. Endocytosis is used for copper nanoparticle adsorption. An investigation of cancer morphology reveals that the production of copper nanoparticles dramatically increases cancer cell apoptosis; therefore, exerting an anti-cancer effect [55]. When chitosan is mixed with copper nanoparticles, the rate of cell death increases substantially. However, copper-based nanoparticles, including copper oxide (CuO), amorphous and crystalline copper sulphide (CuS), copper phosphate (CuPO4), and copper iodide (CuI), have been shown to be cytotoxic to human cancer cells [53].
Pramanik et al. recognized the capacity of copper nanoparticles as an anticancer drug and created a unique copper-based nanoparticle utilizing copper carbonate (CuCO3), which has efficient cytotoxic action [56]. Since CuCO3 nanoparticles are cytotoxic to normal cells, CuCO3 nanoparticles are conjugated with folic acid (FA-CuCO3) to reduce their cytotoxicity and directly target the tumor cells. CuCO3 nanoparticles caused DNA damage and disrupted the mitochondrial membrane, resulting in apoptosis-mediated cell death [57]. To test the targeting efficacy of CuCO3 nanoparticles, shRNA was used to create folate receptor knockdown HeLa cells. In animal models, Pramanik et al. reported that FA-CuCO3 nanoparticles were useful and had the potential to be an effective treatment agent for folate receptor-expressing cancer cells while minimizing cytotoxicity to normal cells [56]. Guo et al. utilized CuO nanoparticles as drug carriers for targeted drug delivery in nasopharyngeal cancer [58]. CuO nanoparticles were loaded with DOX and docetaxel, which are well-established chemotherapeutic drugs that have been used successfully for nasopharyngeal carcinoma. In order to reduce the cytotoxicity of CuO nanoparticles, Guo and co-workers coated CuO nanoparticles with varying amounts of PLGA (polylactide-co-glycolide) and then conjugated them with folic acid to improve tissue targeting [59,60]. The results of the study conveyed that the PLGA coating of CuO nanoparticles changed the surface characteristics of the nanoparticles and improved biodegradation of the nanoparticles within the body while causing no cytotoxicity to normal cells [59,61]. Copper nanoparticles, along with other metallic nanomaterials-based therapeutic treatments and agents, have exhibited considerable maximal efficacies in destroying cancer cells. Nevertheless, more extensive research and investigations are still needed on copper-based nanoparticles to gather accurate data on local toxicity and permanent side effects on the body [61]. Thus, significant preclinical and clinical trials are required before successful translation into clinical use and eventual acquisition of FDA approval [62,63].
3. Organic-Based Nanoparticles
A range of lipid-based carriers with varying physicochemical characteristics and structures, depending on the lipid content and manufacturing procedures, have been used. Most lipid nanocarriers are characterized by their biocompatibility, elevated drug loading competence, stability in vivo, and controlled drug release patterns [12,39]. Liposomes, niosomes, lipid micelles, nano-emulsions, solid lipid nanoparticles, polymeric nanoparticles, chitosan-based nanoparticles, and nanostructured lipid carriers are all examples of lipid-based nanocarriers [14,64,65]. However, the most eminent and significant lipid-based nanocarriers for efficient co-delivery are liposomes and nano-emulsions [14,66].
3.1. Liposomes
Lipid-based nanostructures have been widely utilized in biological applications in the previous decade due to their biological consistency and compatibility. Lipid bilayer membranes assemble phospholipid carriers known as liposomes. Chemotherapeutic agents that are hydrophilic can be encapsulated in the inner aqueous phase, whereas hydrophobic drugs can be contained in the lipid layer [4,13,19]. Hydrophobic drugs have a low encapsulation rate because they are only entrapped in the bilayer interface. Liposomes are mostly made up of phospholipids generated by non-ionic surfactants and other amphipathic chemicals that permit self-assembly [25]. Liposomes are also made up of amphiphilic lipids that form spherical bilayers, similar to the lipid bilayer seen in biological membranes. The increased permeability and retention (EPR) effect generated by the specific anatomical and pathophysiological properties of tumor vasculature causes lipid nanocarriers to accumulate in tumor tissue passively [27,33].
Liposomes can be actively guided for identification by receptors at the tumor location by synthetically altering the liposome surface or terminal PEG (polyethylene glycol) molecule with tumor-specific ligands, such as folic acid, transferrin, or monoclonal antibodies. PEGylation shields the liposome nanocarrier from immune system destruction, thus, increasing the liposome circulation duration [30,33,64]. As a result, the liposome highly accumulates in tumor tissue while decreasing mononuclear phagocyte system absorption. The accumulation of the liposomes at the tumor site can occur either via active or passive targeting. An extra degree of complexity and specificity for the target cell may be accomplished via ligand-mediated targeting, also known as active targeting. After accumulating at the tumor site, drug-carrying liposomes are often taken up intracellularly by endocytosis. The process of endocytosis facilities the liposomes going through endosomes and lysosomes [25,32,39]. Hence, to release the drugs from the liposome intracellularly, the liposome membrane and the cancer cell must merge, or lipid mixing should occur with the endosomal or lysosomal membranes. Drug release can be triggered by external and/or internal mechanisms such as ultrasound, heat, enzymes, pH, etc. AlSawaftah et al. established that ultrasound, at a specific intensity and frequency, induces transient pores, also known as sonoporation, which enhances drug release from the liposomes and drug uptake in cancer cells [66] (Figure 2).
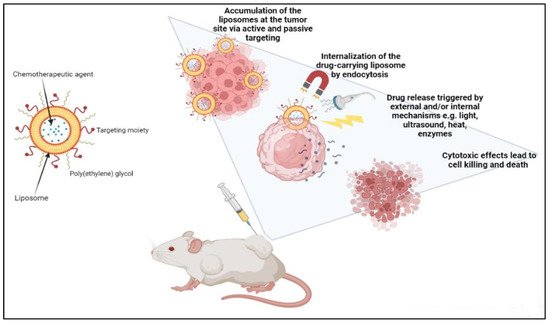
Figure 2. Demonstration of cancer treatment using a liposome-based drug delivery system.
Traditional doxorubicin liposomes, such as Evacet and Myocet, doxorubicin liposomes with prolonged circulation times like Doxil and caelyx, cytarabine liposomes like Depocyt, and paclitaxel liposomes, such as Taxo, are all available on the market [5,27]. Liu et al. created an efficient multilayer liposome vesicle that can prolong the release of DOX and PTX, improving the benefits of the co-delivery system while decreasing systemic toxicity [18]. Sheng et al. employed nanoliposomes to transport perfluorooctyl bromide and ICG for improved multimodal imaging-guided phototherapy [67]. Patel et al. used a thin film hydration approach to create furtive liposomes encapsulated with the P-gp inhibitor tariquidar and the paclitaxel (PTX) [13,32]. An experiment was performed on SKOV-3TR cells, cellosaurus cell line, where the concurrent administration of tariquidar and PTX through prolonged circulating liposomes caused more cytotoxicity in the cancerous cells than administering paclitaxel only, indicating a considerable reversal of the MDR about PTX [13,68,69]. Even though various liposome nano-systems have been presented, liposomes’ limited drug loading capacity and poor stability make them unsuitable for large-scale use [23,25,66,69].
3.2. Lipid Nano-Emulsion (NE)
Nano-emulsion nanocarriers have been explored as an alternative to outweigh the problems with liposome encapsulation efficiency and solubility. Different delivery techniques using nano-emulsion carriers have been utilized as therapeutic vehicles for hydrophobic drugs. Nanodroplets are distributed in a continuous phase in nano-emulsions [18]. Nano-emulsions generally comprise heterogeneous non-miscible liquids sustained by an emulsifying agent or a non-ionic surfactant. Cancer therapy research has shifted towards nano-emulsions because of their crucial properties in attaining effective therapeutic effects [69]. Their high surface area, physical stability, amphiphilicity, longer circulation time, precise targeting, tumor imaging characteristics, and surface functionalization for passive or active targeting allow nano-emulsions to readily concentrate in tumor vasculature and pass through challenging barriers [70,71]. Liu et al. devised a nano-emulsion to enhance chemotherapy delivery and efficacy to lower MDR in nasopharyngeal cancer treatment as an alternative to lowering MDR [13,19,72]. Ultrasonic homogenization was used to make nano-emulsions that efficiently co-delivered DOX and a radiotherapeutic isotope of yttrium-90 (90Y) [14]. The latter system was examined in vivo and in vitro, resulting in a substantial decrease in nasopharyngeal cancer cells. Due to the simultaneous blockage of many pro-tumor pathways, the biological results of a modified nano-emulsion demonstrated a significant reduction in tumor volume in a nude mouse model and an increase in HepG-2 cell death [14,39,69]. Many lipid-based delivery methods have reached the clinical testing phase. Weissig et al. researched and experimented on the co-administration of cytarabine and daunorubicin, also referred to as CPX-351, in patients with acute myeloid leukemia. The latter also investigated CPX-1, which is the encapsulation of irinotecan and floxuridine, specifically in patients with colorectal cancer [71]. The administration of CPX-351 and CPX-1 showed a higher remission rate and a prolonged survival rate in patients. The latter are the two major arrays of liposomal formulations of small molecule drug combinations that have commenced clinical trials [73,74,75]. Table 1 summarizes various nanomedicines currently under clinical trials for cancer treatment.
Lipid nanocarriers are commonly employed in co-delivery systems because of their varied hydrophilicity and low cytotoxicity; nevertheless, they might be unstable in systematic circulation and have a limited half-life in the body [13,14]. Manufacturing hybrid nanoparticles that combine lipids with other materials, such as synthetic polymers or inorganic material, is another way to reduce colloidal instability [76,77].
Table 1. Nanomedicines undergoing clinical trials for cancer treatment.
| Commercialized Formulation (Active Ingredient) |
Nanocarrier Type | Indications | Company | Clinical Trial Phase | Reference |
|---|---|---|---|---|---|
| Onco-TCS (Vincristine) |
Liposomes | Non-Hodgkin Lymphoma | INEX Pharmaceuticals | Clinical phase 1/2 | [78] |
| OSI-211 (Lurtotecan) |
Liposomes | Lung cancer Recurrent ovarian cancer |
OSI | Clinical phase 2 | [79] |
| LEP-ETU (Paclitaxel) |
Liposomes | Ovarian, breast, and lung cancers | Neopharma | Clinical phase 1/2 | [78] |
| Auroshell | Gold-silica nanoshells | AuroLase therapy for cancer | Nanospectra Biosciences | Clinical phase 1 | [80] |
| Thermodox (Doxorubicin) |
Liposomes | Hepatocellular carcinoma | Celsion | Clinical phase 3 | [78] |
| Aroplatin (Cisplatin analog) |
Liposomes | Colorectal cancer | Antigenics, Inc. | Clinical phase 1/2 | [79] |
| Nektar-102 (PEGylated irinotecan) |
Liposomes | Breast, colorectal cancer | Nektar therapeutics | Clinical phase 3 | [78] |
| NKTR-105 (PEG-Docetaxel) |
Polymer-drug conjugate | Solid tumors | Nektar therapeutics | Clinical phase 1 | [80] |
| CYT-6091 Aurimmune (TNF-α) |
TNF-α bound to colloidal gold nanoparticles | Head and neck cancer | Cytimmune Sciences | Clinical phase 2 | [78] |
| Paclical (Paclitaxel) |
Polymeric micelles | Ovarian cancer | Oasmia Pharmaceutical AB | Clinical phase 3 | [79] |
| Lipoplatin (Cisplatin) |
Liposomes | Pancreatic, head and neck, breast cancer | Regulon | Clinical phase 3 | [78] |
3.3. Polymeric Micelles
Polymer-based delivery systems are one of the most common platforms for delivering chemotherapeutic agents in co-delivery systems [19]. Polymer-based nanocarriers that load and distribute anti-tumor agents have piqued attention and become a focus for research to get around the limitations of the single delivery of chemotherapeutic agents. Polymer-based delivery systems are novel drug delivery systems that are prominently stable and reliable alternatives to lipid-based carriers [8,21]. Natural and synthetic polymers have been used to create polymeric nanocarriers [26]. Polymer-based micelles, polymersomes, polymer nanoparticles (NPs), nanogels, and mixed polymeric nanoparticles with porous cores are the five main forms of polymer nanocarriers [21,24].
Polymeric micelles are spherical amphiphilic, biodegradable, and flexible nanoparticles made by combining various amphiphilic di-block or tri-block copolymers. In an aqueous medium, the polymeric micelles have hydrophilic and hydrophobic blocks [80]. The hydrophobic micelle cores serve as a reservoir for hydrophobic drug encapsulation, whereas the hydrophilic shells can capture hydrophilic molecules [15,33]. There are two basic mechanisms the polymeric micelle follows to release the encapsulated drugs. The micelle dissociates, and then the drug separates by breaking down from monomers. Then, the polymer of the drug links inside the micelle, followed by a diffusional discharge from the micelle core nanocarrier (Figure 3) [25,33]. In cancer therapy, polymeric micelles, rather than lipid micelles, have been used in several studies as optimal delivery vehicles for different therapeutic agents. Polymeric micelle nanocarriers reflect a particular interest in drug delivery applications because of their versatile characteristics, such as low cost, ease of synthesis, ease of conjugation of functional groups, biocompatibility, and biodegradability, among other characteristics [4,6,81].
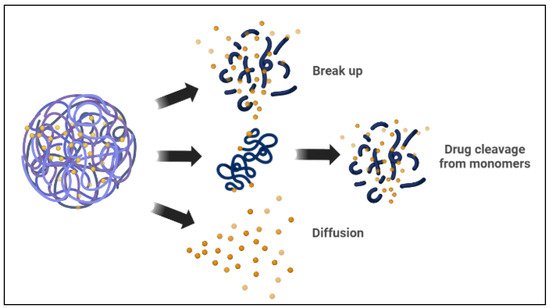
Figure 3. Drug release mechanisms from polymeric micelles.
With their ability to reduce MDR and increase the efficacy of co-delivery systems, these nanocarriers are being investigated more extensively [69,77]. Lv et al. have developed a new polymeric micelle to co-deliver DOX and curcumin (CUR) for MCF-7/ADR cells [14]. MCF-7/ADR cells have been frequently used in cancer research as a multidrug-resistant breast cancer cell type [81,82]. Lv et al. have developed a poly-(ethylene glycol)-block-poly-(lactide) (PEG2k-PLA5k) polymeric micelle and demonstrated an increase in the therapeutic efficacy of the co-delivery systems against MCF-7/ADR cells [14,83]. The approach used by Carvalho et al. demonstrated that the co-delivery system could generate increased tumor accumulation and good anti-tumor effects in MCF-7/ADR cells compared to single drug delivery using this method [65]. For the same drug combination of DOX and CUR, Zhang et al. developed methoxy poly (ethylene glycol) poly(caprolactone) micelles [26,81,84]. The latter co-delivery system sustained a suitable plasma concentration of active drugs by extending blood circulation and possessed slow-release characteristics [83]. Lin et al. created a multi-functional block copolymer micelle for the co-delivery of paclitaxel (PTX) and siRNA, a combination of an anti-cancer drug and a gene [81,85]. The latter micelle is unique in its ability to selectively deliver a combination of therapeutic agents. It has several important characteristics, including self-assembly, passive tumor targeting, and amplified cell death [86,87]. Furthermore, Wang et al. developed a new carrier for in vitro studies made up of PEG micelles and poly(e-caprolactone) (PCL) for the co-delivery of both PTX and an MDR reversal drug, commercially known as tacrolimus [74,88]. PEG-PCL micelles were found to reduce MDR effects by promoting apoptosis in human ovarian cancer cells by directly stimulating the apoptosis signaling system [14,21,84,88].
3.4. Cubosome Nanoparticles
Cubosomes are self-assembled liquid crystalline particles, specifically created from the lipid cubic phase and stabilized by an outer corona, which is polymer based and can be employed for targeting [88]. A single lipid bilayer generates a continuous periodic membrane lattice structure with holes generated by two interlaced water channels in lipid cubic phases. The latter microstructure gives the cubosome distinctive features of practical significance, such as stability under different physiological settings. Cubosomes are considered a unique discovery that traverses the areas of three-dimensional geometry, organic membranes, and digestive processes [89]. The structure has a substantially larger membrane surface area than liposomes for loading membrane proteins and small molecule drugs. The cubic phases have generated a lot of interest because their unique architecture is medically friendly and capable of regulating the release of drugs, proteins, and other solubilized active components [90]. Cubosomes have different forms, in bulk or as nanoparticles. The latter has extensive potential applications ranging from drug delivery to membrane bioreactors, artificial cells, and biosensors.
Most studies on loaded cubosomes have been of proteins or small molecule drugs integrated inside the lipid membrane, with the use of single or binary lipid compositions based on monoolein or phytantriol. There have been multiple studies of cubosomes being loaded with small molecule drugs, including chemotherapeutic drugs for cancer treatment [91]. For small molecule chemotherapeutic drugs, studies show encapsulation effectiveness that ranges from 71 to 103 percent [90,92]. The capacity to create improved encapsulation and delivery vectors is critical to the success of gene technologies [92]. Cubosomes, when compared to a liposomal equivalent, contain a greater amount of siRNA. Notably, the cubosome membranes contain inherent fusogenic characteristics that encourage endosomal escape. Despite their considerable potential, typical cubosome formation approaches result in particle sizes that are too big to meet the advanced standards of delivery vectors. Therefore, Kim et al. utilized a microfluidic nanomanufacturing system to create cubosomes and siRNA-loaded cubosomes, known as cuboplexes [93]. Kim et al. successfully enabled the synthesis of tiny cubosomes and cuboplexes (75 nm) that surpassed the performance of commercially available delivery vectors, such as liposome-based arrangements, by using cryogenic TEM and small angle X-ray scattering through microfluidic devices [91,94]. Drug delivery, and specifically the co-delivery of multiple drugs, is now the most important biomedical use of cubosomes, since several of the fundamental elements have previously been approved for medical use. Since several drugs and therapeutic agents have limited solubility and permeability, lipid carriers are an appealing choice for drug delivery. While tremendous progress has been achieved in the past five years, there are currently no FDA-approved cubosome articulations for theragnostic purposes on the market; however, initial in vivo studies appear encouraging [88].
3.5. Polymersomes
Polymersomes are self-assembled artificial vesicles that are empty at the center; thus, they have a structural pattern that is comparable to that of polymer liposomes [12]. Polymersomes are constructed from synthetic amphiphilic block copolymers such that hydrophilic molecules are in the aqueous solution-filled core, whereas the hydrophobic molecules are located within the membrane. Polymersomes are considered to have one of the most intriguing architectures for drug delivery systems because of their membrane resiliency [4,68,85]. These polymersomes can readily release drugs from external stimuli, such as ultrasound, light, and magnetic fields. For the targeted drug delivery system against the A549 cell line in lung cancer, polymersome nanoparticles were designed to load DOX and RNA aptamer, an epithelial cell adhesion molecule [30,95]. The polymersome was made up of FDA-approved polyethylene glycol and poly(lactic-co-glycolic) acid copolymer for the regulated drug delivery in A549 cells [21,27]. The targeted nanocarrier demonstrated low cytotoxicity through in vitro and in vivo assays. In addition, the targeted nanocarrier improved therapeutic effectiveness and had a higher inhibition of cancer cell proliferation when compared to non-targeted single drug delivery [64,74,95]. Table 2 presents selected clinically approved and marketed nano-drugs for cancer therapy.
Currently, significant attention has been directed to polymeric nanoparticles as innovative carriers for the majority of co-delivery systems [14,96]. The ability of the polymeric nanoparticles to carry hydrophobic and hydrophilic drugs together, as well as their beneficial ability to adequately control drug release, has made them favorable carriers [20,69]. In addition, polymeric nanocarriers possess advantageous characteristics, such as minimal systemic toxicity, high stability, and long circulation time, all of which help them accumulate in the tumor microenvironment. There have been several research and review papers published on cancer treatment by the co-delivery of different chemotherapeutic drugs via polymeric nanoparticles [20,23,30,95].
Table 2. Clinically approved and marketed nano-drugs for cancer.
| Nanomaterial Type | Common Trade Name | Composition | Delivery | Indication | Approval Date | Company | Reference |
|---|---|---|---|---|---|---|---|
| Lipid-based nanoparticles | Doxil®/Caelyx® | PEGylated liposomal doxorubicin | Non-targeted delivery Immunoevasion |
Metastatic ovarian, breast cancer, multiple myeloma, HIV-associated Kaposi’s sarcoma (KS) | FDA—1995 EMA—1996 |
Orthobiotech/Schering-Plough Canada Inc. Janssen-Cilag, Europe |
[97,98,99] |
| Lipodox® | FDA—2013 | Sun Pharmaceutical Industries Ltd. (SPIL) | [97,100] | ||||
| DepoCyt® | Liposomal cytarabine | Non-targeted delivery | Lymphomatous meningitis | FDA—1999 | Skye Pharma, Enzon | [77] | |
| DaunoXome® | Liposomal daunorubicin | Non-targeted delivery | HIV-associated Kaposi’s sarcoma (KS) |
FDA—1996 | Galen Ltd., USA/Gilead Science, Inc., Ireland | [78,101] | |
| Onivyde® | PEGylated liposomal irinotecan | Non-targeted delivery Immunoevasion |
Metastatic pancreatic cancer | FDA—2015 | Merrimack Pharmaceuticals Inc., Massachusetts, USA | [99,102] | |
| Lipid-based nanoparticles | Myocet® | Non-PEGylated liposomal doxorubicin | Non-targeted delivery | Breast cancer | EMA—2000 (Approved in Europe and Canada) | Enzon Pharmaceuticals for Cephalon in Europe Elan Pharmaceuticals/Sopherion Therapeutics in Canada |
[103,104,105] |
| Mepact® | Liposomal mifamurtide | Non-targeted delivery | Osteogenic sarcoma | EMA—2009 (Approved in Europe) | Takeda France SAS | [101,106] | |
| Marqibo® | Liposomal vincristine sulfate | Non-targeted delivery Sustained Release |
Acute lymphoblastic leukemia | FDA—2012 | Talon Therapeutic, Inc., California, USA | [102] | |
| Lipid-based nanoparticles | Lipusu® | Liposomal paclitaxel | Non-targeted delivery Sustained Release |
Breast cancer, NSCLC, ovarian cancer | Approved in China—2006 | Luye Pharma Group | [107,108,109] |
| Vyxeos® | Liposomal daunorubicin and cytarabine | Combinatorial delivery | Acute myeloid leukemia | FDA—2017 EMA—2018 |
Jazz Pharmaceutics, Inc. | [106,110] | |
| Polymer-based nanoparticle | Genexol-PM® | Paclitaxel micellar | Sustained Release | Breast, ovarian, gastric cancer, and NSCLC | Approved in South Korea—2007 | Samyang, Seongnam, South Korea | [111,112] |
| Eligard® | Leuprolide acetate and polymer | Non-targeted delivery | Prostate cancer | FDA—2002 | Tolmar Pharmaceuticals Inc. | [113,114] | |
| Protein- drug conjugate | Pazenir® | Paclitaxel | Non-targeted delivery | Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, NSCLC | EMA—2019 | Ratiopharm GmbH San Francisco, CA, USA |
[107] |
| Oncaspar® | PEGylated L-asparaginase conjugate | Non-targeted delivery | Acute lymphocytic leukemia | FDA—1994 | Enzon Pharmaceuticals Inc. | [114,115,116] | |
| Protein nanoparticle | Abraxane® | Albumin-bound paclitaxel | Non-targeted delivery Sustained Release |
Metastatic breast cancer Lung cancer, and NSCLC |
FDA—2005 EMA—2008 FDA—2012 |
Abraxis Bioscience, AstraZeneca | [117,118,119] |
| Nab-paclitaxel in combination with gemcitabine | Metastatic pancreatic adenocarcinoma | FDA—2013 | Celgene Pharmaceutical Co. Ltd. | ||||
| Inorganic nanoparticle | Hensify® | Hafnium oxide nanoparticles | Non-targeted delivery Radiation-activated |
Locally advanced squamous cell carcinoma | EMA—2019 | Nanobiotix | [110] |
| Nano-therm | Iron oxide (Fe2O3) | Hyperthermia | Glioblastoma, prostate, and pancreatic cancer. | FDA—2010 EMA—2013 |
Magforce | [102] |
Abbreviations: FDA: Food and Drugs Administration; EMA: European Medicines Agency; NSCLC: non-small cell lung; PEG: polyethylene glycol.
This entry is adapted from the peer-reviewed paper 10.3390/nano12152672
This entry is offline, you can click here to edit this entry!
