Seagrass plays a significant role in aquatic environment acting as an animal shelter and breeding place, oxygen and nutrients supplier, as well as pollutant controller. The loss or degradation of seagrass ecosystem could impact human lives, especially for people residing in coastal area. However, seagrass conservation efforts are considered insignificant, both at the national as well as international level. This problem might be arisen due to the lack of direct use of seagrass by humans, and limited number of related studies. This paper reviews the potential of seagrass species in Indonesia by using Google Scholar database. From 12 species of seagrass in Indonesia, only Endalus acoroides, Cymodocea serrulate, and Syringodium isoetifolium that have been reported for its direct utilities from at least 5 research papers. The result of this study shows bioactivities of anticancer, antibacterial, antioxidant, and antifoulant from various seagrass species which could be used in commercial purposes to increase the economic value of the community. Furthermore, a significant finding of novel antibacterial and anticancer activities from C. serrulate could contribute to the advancement of pharmaceutical and medicinal research. By highlighting the commercial use potentials of seagrass, we expect to persuade all related stakeholders to realize the seagrass conservation.
- seegrass
- conservation
- Indonesia
- pharmaceutical
INTRODUCTION
Seagrass ecosystem is considered important due to its role of supporting the survival of aquatic species in all trophic levels. Seagrass is an underwater plant species that makes up most of the grasslands, have evolved from terrestrial and back into the sea millions of years ago. Its roles are not only in compiling aquatic habitats, but also through complex ecosystems; contribute to the well-being of coral reefs, mangroves, salt marshes, and oyster reefs [1][2]. The ecosystem of seagrass can be overgrown by one or up to 8 different species. Seagrass provide nutrients (N and P) and organic carbon both for inhabitants of seagrass ecosystem as well as the other part of the ocean, including the deep sea, while contributing in carbon sequestration [3]. Apart from being sources of food, seagrass ecosystems also play an important role as a shelter for larvae and baby fish or shellfish [2][4].The role seagrass in aquatic ecosystem has been summarized in an illustration presented below (Figure 1).
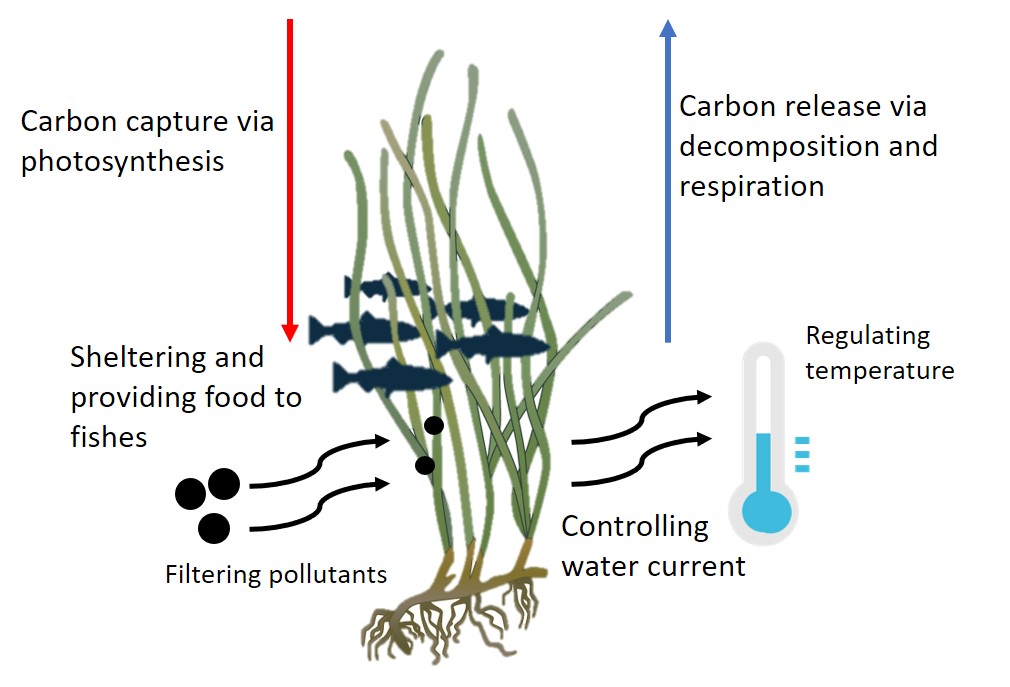
Unfortunately, those vital roles cannot prevent seagrass from extinction. Costanza et al. [5] (converted by Short et al. [6]) explained that the value of seagrass ecosystem could reach USD 34,000 per hectare per year which is higher than of other terrestrial and marine habitats. Several reviews show that there is a reduction in seagrass areas [6][7][8]. Waycott et al. [8] with their synthesis study, reported the rate of decline in seagrass areas has reached 110 km2 annually between 1980 and 2006. A total of 72 seagrass species, after a long-time negligence, were later listed on the International Union for the Conservation of Nature (IUCN) red list of endangered species.
Then, how is the condition of seagrass ecosystem in Indonesia? Seagrass covers at least 30,000 km2 of Indonesian ocean spreading from Sabang to Merauke. Thus, it is no wonder that Indonesia has been named one of the countries with the highest seagrass diversity in the world. Seagrasses that grow in Indonesia are classified into 7 genera and 12 species. However, seagrass ecosystems in Indonesia are threatened by many factors, from overfishing to hazardous material pollution [9]. Moreover, the lack of attention from the government and non-government organizations (globally) has exacerbated seagrass conservation efforts [10]. Research conducted by Kadir et al. [11] shows that at least the damage suffered by seagrass ecosystems in Indonesia has reached 40%. Based on a review conducted by Unsworth et al. [12], at least 75% of marine conservationists have reported a significant reduction of seagrass ecosystems during the last 5 years (2013-2018), while another 12% reported no changes in the coverage of seagrass ecosystems. The review by Unsworth et al. [12] reported coastal development to be the main cause of seagrass loss (17%), while other causes include reclamation (12.5%), deforestation-driven sedimentation (8%), seaweed cultivation (8%), coral mining (8%).
The direct impacts of seagrass extinction on humans include the loss of income and food sources. Fish catching and collecting activities have decreased dramatically as the seagrass ecosystem decreased (i.e mussel collection in East Africa [13]). In countries with high demand for seafoods, such as Korea, Japan, and China, seagrass species are listed as endangered (such as Phylospadix iwatensis, Phylospadix japonicus, Zostera asiatica, Zostera caespitosa, Zostera caulescens, and Zostera geojeensis) on the basis that seagrass holds significant impact on the fisheries sector in those countries [14]. This is due to the fact that seagrasses are a breeding ground for many important commercial fish species. In addition, reduction or even extinction of seagrass ecosystems will significantly reduce fish quality, water quality, shoreline stability, and ecosystem richness [3].
Despite its significant impact on humans, seagrass conservation efforts are conducted minimally. This may be due to the impact occurs slowly, so it is not really felt by humans. Therefore, this paper provides a different perspective on the potential of seagrass direct uses; which can be commercialized. Several studies have reported this potential, but only limited to a few species. This paper will provide information related to the distribution of seagrass species in Indonesia so that we can easily map their potential utilization. It is hoped that by providing this new perspective, it will promote the importance of seagrass conservation.
MATERIAL AND METHODS
In this work, we compiled reports from Google Scholar database that include the seagrass species exploration in Indonesian waters, followed by further search on the utility of the seagrasses worldwide. The search was conducted on December 30th, 2021. To search the seagrass species the following terms were used: (Seagrass OR lamun) AND (Indonesia). Following that, the utility or application each seagrass was searched using the name of the species along with their known synonyms. All review articles (in any types) were excluded. Articles should be written in English or Indonesian language; otherwise, the articles were excluded.
RESULT AND DISCUSSION
Seagrass Species in Indonesia
The distribution of seagrass in Indonesian waters has been extensively discussed by Kuriandewa et al., [15]. The report suggested that Enhalus acoroides, Halophila ovalis, Thalassia hemprichii, Cymodocea rotundata, Halodule uninervis, and Syringodium isoetifolium were found in almost all studied locations. The aforementioned research is more specific on 4 sampling points: Riau Islands, Seribu Islands, Talaud Islands, and Tanimbar Islands. Another study found that T. Hemprichii and C. Rotundata had a greater broad coverage than other seagrass species observed [9]. The reason why certain species have high distribution is due to their high adaptability allowing them to tolerate significant changes of water condition in wider area [16]. Density and percentage of wide coverage in each area was found to be varied, attributed to the influence of the substrate of seagrass growing media [17]. In addition, there is also a newly reported species, Halophila sulawesi, which is similar to H. ovalis, but monoic [18].
Commercial Potential of Seagrass
Based on our search in Google Scholar database, direct uses of seagrass are still scarcely reported. Most of the studies investigated antioxidant and antibacterial potentials derived from the secondary metabolites of a seagrass. Several pharmacological properties were found in E. acoroides, H. decipiens, H. ovalis, T. hemprichii, C. serrulata, H. uninervis, and S. isoetifolium. Amudha et al [19] reported that E. acoroides has high content of phenols and flavonoids that acts as antioxidants. Similar results were also reported by Dewi et al (2017) who conducted research on n-hexane extract from E. acoroides. Moreover, Kannan et al. [20] investigated leaves, rhizomes, and roots of E. acoroides that revealed high antioxidant activities of the seagrass extracts. In addition, bioactivities of E. acoroides as an antifeedant, antibacterial and anti-larvae has also been reported by Qi et al [21]. Apart from its secondary metabolites that could be used as medicines, E. acoroides could also be consumed as food, which is commonly practiced in the Philippines [22][23].
C. serrulata has been investigated for various applications, including: antibacterial agent [1][2], antifoulant [3], bio-reductor for synthesis of nanoparticles [3], and anti-cervical cancer agent [4]. It has been tested as an antibacterial agent against various human pathogens (S. aureus, Bacillus cereus, Bacillus subtilis, E. coli, Salmonella paratyphi, Salmonella typhimurium, and Microccus luteus) by Kumar et al. [1]. More interestingly, Gnanambal et al., [2] have successfully isolated a novel antibacterial phenyl thioketone from C. serrulata. These antibacterial agents are able to carry out antibacterial activity effectively against gram-positive and negative isolates that have shown resistance to commercial drugs. This shows that the exploration of new compounds in seagrass plants could provide an alternative to multidrug-resistant bacterial infection. Seagrass extract have also been reported for its role in inhibiting cervical cancer cells development [4]. The synthesis of silver nanoparticles by Palaniappan et al. [3] using C. serrulata extract confirmed its potential as a treatment for lung cancer. Additionally, C. serrulata has also been reported to have a potential application as an antifoulant [5], therefore can be utilized in the marine industry.
Antifouling activity was also found in S. isoetifolium which can also inhibit the reproduction of brown mussels (Perna indica) [6]. Its toxicity to bacteria and insects has also been reported by Mani et al (2012) employing methanol extract from S. isoetifolium. The methanol extract, based on phytochemical test, contains compounds from the saponins, resins, proteins, carbohydrates, glucosides, acid compounds, reducing sugars, cardiac glucosides, phenols and alkaloids. S. isoetifolium lotion cream for human skin also has been reported by Juwita et al [8]. Aside from its application on human, ethanol extract from the leaves and root of S. isoetifolium also effectively inhibits pathogen bacteria in fish (Aeromonas hydrophila) [7].
Another seagrass species, H. uninervis, is reported to have antidiabetic activity [9] and can be utilized in the synthesis of silver nanoparticles [10]. Karthikeyan and Sundarapandian [11] reported the antidiabetic activity of H. uninervis methanol extract and the presence of phytochemical saponins, glycosides, steroids, and phenolics. These secondary metabolites are contained the methanol extract from H. uninervis that is associated with its capability of reducing blood sugar levels as well as reactive oxygen species in the tested mice. In addition, the potential utilization of H. uninervis can be seen in research of Mahyoub et al. [10] who synthesized silver nanoparticles using its extracts. Silver nanoparticle that produced from eco-friendly synthesis then applied as Aedes aegypti larvicide, which considered threatening human life quality because it can be the virus vector dengue and zika fever.
Furthermore, H. decipiens, H. ovalis, T. hemprichii, and C. rotundata, each of them were reported by only one research study. Antioxidant activity was found in H. decipiens [12] and T. hemprichii [13]. Based on Dewi et al.,[14] although T. hemprichii showed antioxidant activity with secondary metabolites containing flavonoids, alkaloids and steroids, the toxicity level was found high with an LC50 value of 5.74 mg/L. Meanwhile, two other seagrass species, H. ovalis and C. rotundata, possess antibacterial properties that have been tested on Staphylococcus aureus and Escherichia coli [1][15]. The other three seagrass species from Indonesia, H. minor, H. spinulosa, and T. cilitatum, have never been reported for its direct use. The limited number of publications on certain seagrass species may be due to the rarity of these species in Indonesian waters. However, this also shows the importance of conserving seagrass species for there are still many seagrass potentials that have not been studied.
Table 1. Seagrass species in Indonesia and their direct utilities
|
Seagrass species in Indonesia |
Industrial sector |
Commercial application |
|
E. acoroides |
Pharmaceutical |
Antioxidant |
|
Antifeedant, antibacterial and anti-larvae |
||
|
Food |
Food ingredient |
|
|
C. serrulata |
Pharmaceutical |
Antibacterial |
|
Anti-cervical cancer |
||
|
Anti-lung cancer |
||
|
Chemical/pharmaceutical |
Bio-reductant |
|
|
Marine |
Antifoulant |
|
|
S. isoetifolium |
Pharmaceutical |
Anti-diabetic |
|
Cosmetics/ pharmaceutical |
Lotion cream |
|
|
Chemical/agriculture |
Insecticide |
|
|
Marine |
Antifoulant |
|
|
Fisheries |
Anti-fish-related pathogen |
|
|
H. uninervis |
Pharmaceutical |
Anti-diabetic |
|
Chemical/pharmaceutical |
Bio-reductant, anti-larvae |
|
|
H. decipiens |
Pharmaceutical |
Antioxidant |
|
T. hemprichii |
Pharmaceutical |
Antioxidant |
|
H. ovalis |
Pharmaceutical |
Antibacterial |
|
C. rotundata |
CONCLUSION
The threat of seagrass ecosystem coverage decrement, border-lined extinction, also have been reported in Indonesian water that was initially rich in seagrass species. There are at least 12 seagrass species (with one additionally new species) in Indonesia; E. acoroides, H. decipiens, H. minor, H. ovalis, H. spinulosa, T. hemprichii, C. rotundata, C. serrulata, H. pinifolia, H. uninervis, S. isoetifolium, dan T. cilitatum. Overall, the studies on the direct utility of seagrass species inhabiting Indonesian waters are still scarce. Published researches dominated by its use in pharmaceutical sector such as antibacterial, antioxidant, anticancer, and antidiabetic. Seagrass direct use as comestibles only reported for E. acoroides species which is widely found in Indonesia. As for other direct uses, seagrass extracts have been tested for their ability as antifouling and insecticide agents. Moreover, H. minor, H. spinulosa, H. pinifolia and T. cilitatum have not been investigated for their direct use. The fact that seagrass species in Indonesia are still unknown for their potentials further urges the importance of seagrass conservation.
References
Amudha, P. et al. 2017. Phytochemical Analysis and Invitro Antioxidant Screening of Sea grass-Enhalus acoroides. International Journal of Research in Pharmaceutical Sciences. 8(2):251–258.
Chanthini, A. B. et al. 2015. Structural Characterization, Antioxidant and In Vitro Cytotoxic Properties of Seagrass, Cymodocea serrulata (R.Br.) Asch. & Magnus Mediated Silver Nanoparticles’. Journal of Photochemistry and Photobiology B: Biology. 153:145–152.
Costanza, R. et al. 1996. The Value of The World’s Ecosystem Services and Natural Capital. Nature. Report of Workshop organised by NCEAS, Santa Barbara, Calif. p: 387.
Dewi, C. S. U., Soedharma, D. &Kawaroe, M. 2017. Komponen Fitokimia dan Toksisitas Senyawa Bioaktif dari Lamun Enhalus acoroides dan Thalassia Hemprichii danri Pulau Pramuka, DKI Jakarta. Jurnal Teknologi Perikanan dan Kelautan. 3(2): 23–27.
Dorenbosch, M. et al. 2004. The Relationship of Reef Fish Densities to The Proximity of Mangrove and Seagrass Nurseries. Estuarine, Coastal and Shelf Science. 60(1): 37–48.
Duarte, C. M., Middelburg, J. J. & Caraco, N. 2005. Major Role of Marine Vegetation on The Oceanic Carbon Cycle. Biogeosciences. 2(1): 1–8.
Gavin, N. M. & Durako, M. J. 2011. Localization and Antioxidant Capacity of Flavonoids from Intertidal and Subtidal Halophila johnsonii and Halophila decipiens. Aquatic Botany, 95(3): 242–247.
Gnanambal, K. M. E., Patterson, J. & Patterson, E. J. K. .2015. Isolation of a Novel Antibacterial Phenyl Thioketone from the Seagrass, Cymodocea serrulata. Phytotherapy Research, 29(4): 554–560.
Iyapparaj, P. et al. 2013. Antifouling Activity of The Methanolic Extract of Syringodium isoetifolium, and Its Toxicity Relative to Tributyltin on The Ovarian Development of Brown Mussel Perna indica. Ecotoxicology and Environmental Safety. 89: 231–238.
Iyapparaj, P. et al. 2014. Antifouling and Toxic Properties of The Bioactive Metabolites from The Seagrasses Syringodium isoetifolium and Cymodocea serrulata. Ecotoxicology and Environmental Safety. 103: 54–60.
Jr., K. L. H. and Hays, G. 2003. Critical Evaluation of The Nursery Role Hypothesis for Seagrass Meadows. Marine Ecology Progress Series. 253: 123–136.
Juwita, A. P., Yamlean, P. V. Y. and Edy, H. J. 2013. Formulasi Krim Ekstrak Etanol Daun Lamun (Syringodium isoetifolium). Pharmacon, 2(2): 8–13.
Kadir, N. et al. 2011. Challenging for Seagrass Management in Indonesia’, J Coast Dev, 15.
Kaewsrikhaw, R. and Prathep, A. 2014. The Effect of Habitats, Densities and Seasons on Morphology, Anatomy and Pigment Content of The Seagrass Halophila ovalis (R.Br.) Hook.f. at Haad Chao Mai National Park, Southern Thailand. Aquatic Botany. 116: 69–75.
Kannan, R. R. R., Arumugam, R. and Anantharaman, P. 2010. In Vitro Antioxidant Activities of Ethanol Extract from Enhalus acoroides (L.F.) Royle. Asian Pacific Journal of Tropical Medicine. 3(11): 898–901.
Karthikeyan, R. and Sundarapandian, M. 2017. Antidiabetic Activity of Methanolic Extract of Halodule uninervis in Streptozotocin-Induced Diabetic Mice. Journal of Pharmaceutical Sciences and Research. 9(10): 1864–1868.
Kawaroe, M. et al. 2016. Seagrass Biodiversity at Three Marine Ecoregions of Indonesia: Sunda Shelf, Sulawesi Sea, and Banda Sea. Biodiversitas, 17(2): 585–591.
Keast, A. 2000. The Ecology of The Indonesian Seas. Part I. The Ecology of Indonesia Series, Volume VII . Tomas Tomascik , Anmarie Janice Mah , Anugerah Nontji , Mohammad Kasim Moosa The Ecology of the Indonesian Seas. Part II. The Ecology of Indonesia Series, Volume VII. The Quarterly Review of Biology, 75(2): 201–201.
Kumar, C. S. et al. 2008. Antibacterial Activity of Three South Indian Seagrasses, Cymodocea serrulata, Halophila ovalis and Zostera capensis. World Journal of Microbiology and Biotechnology. 24(9): 1989–1992.
Kuo, J. 2007. New Monoecious Seagrass of Halophila sulawesii (Hydrocharitaceae) from Indonesia. Aquatic Botany. 87(2): 171–175.
Kuriandewa, T. E. et al. 2003. The Seagrasses of Indonesia. In: World Atlas of Seagrasses. UNEP-WCMC. California. p: 171–181.
Latuihamallo, Y., Watuguly, T. and Tuapattinaya, P. M. 2019. Kualitas Susu Berbahan Dasar Biji Lamun Jenis Enhalus acoroides: Penentuan Nilai Viskositas dan Pengujian Sifat Mikrobiologi di Laboratorium. Biopendix: Jurnal Biologi, Pendidikan dan Terapan. 5(2): 119–129.
Mahyoub, J. A. et al. 2017.Seagrasses as Sources of Mosquito Nano-Larvicides? Toxicity and Uptake of Halodule uninervis-Biofabricated Silver Nanoparticles in Dengue and Zika Virus Vector Aedes aegypti. Journal of Cluster Science. 28(1): 565–580.
Mani, A. E., Aiyamperumal, V. and Patterson, J. 2012. Phytochemicals of The Seagrass Syringodium isoetifolium and Its Antibacterial and Insecticidal Activities. European Journal of Biological Sciences. 4(3): 63–67.
Montaño, M. N. E., Bonifacio, R. S. and Rumbaoa, R. G. O. 1999. Proximate Analysis of The Flour and Starch from Enhalus acoroides (L.f.) Royle seeds. Aquatic Botany. 65(1–4): 321–325.
Ooi, J. L. S. et al. .2011. Knowledge Gaps in Tropical Southeast Asian Seagrass Systems. Estuarine, Coastal and Shelf Science. 92(1): 118–131.
Orth, R. et al. 2006. A Global Crisis for Seagrass Ecosystems. BioScience. 56: 987–996.
Palaniappan, P., Sathishkumar, G. and Sankar, R. 2015. Fabrication of Nano-Silver Particles Using Cymodocea serrulata and Its Cytotoxicity Effect Against Human Lung Cancer A549 Cells Line. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 138: 885–890.
Qi, S.-H. et al. 2008. Antifeedant, Antibacterial, and Antilarval Compounds from The South China Sea Seagrass Enhalus acoroides. Botanica Marina, 51(5).
Ravikumar, U., Vinoth, R. and Selvan, G. P. 2011. Bioactive Potential of A Seagrass Syringodium isoetifolium Against Bacterial Fish Pathogens. Journal of Pharmacy Research. 4(6): 1854–1856.
Septiani, S., Dewi, E. N. and Wijayanti, I. 2017. Antibacterial Activities of Seagrass Extracts (Cymodocea rotundata) Against Staphylococcus aureus and Escherichia coli. SAINTEK PERIKANAN : Indonesian Journal of Fisheries Science and Technology. 13(1): 1.
Shi, Y. et al. 2010. Overview on Seagrasses and Related Research in China. Chinese Journal of Oceanology and Limnology. 28(2): 329–339.
Short, F. T. et al. 2011. Extinction Risk Assessment of The World’s Seagrass Species. Biological Conservation. 144(7): 1961–1971.
Unsworth, R. K. F. et al. 2018. Indonesia’s Globally Significant Seagrass Meadows are Under Widespread Threat. Science of The Total Environment. 634: 279–286.
Unsworth, R. K. F. and Cullen, L. C. 2010. Recognising The Necessity for Indo-Pacific Seagrass Conservation. Conservation Letters. 3(2): 63–73.
Watson, R., Coles, R. and Lee Long, W. 1993. Simulation Estimates of Annual Yield and Landed Value for Commercial Penaeid Prawns from A Tropical Seagrass Habitat, Northern Queensland, Australia. Marine and Freshwater Research. 44(1): 211.
Waycott, M. et al. 2007. Vulnerability of Seagrass in The Great Barrier Reef to Climate Change. ECU Publication.
References
- Chinnadurai Sreenath Kumar; Dronamraju V. L. Sarada; Thomas Paul Gideon; Ramasamy Rengasamy; Antibacterial activity of three South Indian seagrasses, Cymodocea serrulata, Halophila ovalis and Zostera capensis. World Journal of Microbiology and Biotechnology 2008, 24, 1989-1992, 10.1007/s11274-008-9695-5.
- K. Mary Elizabeth Gnanambal; Jamila Patterson; Edward J. K. Patterson; Isolation of a Novel Antibacterial Phenyl Thioketone from the Seagrass,Cymodocea serrulata. Phytotherapy Research 2015, 29, 554-560, 10.1002/ptr.5283.
- P. Palaniappan; G. Sathishkumar; Renu Sankar; Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 138, 885-890, 10.1016/j.saa.2014.10.072.
- Abdhul Basheer Chanthini; Govindasamy Balasubramani; Rajendiran Ramkumar; Rajamani Sowmiya; Manickam Dakshinamoorthi Balakumaran; Pudhupalayam Thangavelu Kalaichelvan; Pachiappan Perumal; Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R.Br.) Asch. & Magnus mediated silver nanoparticles. Journal of Photochemistry and Photobiology B: Biology 2015, 153, 145-152, 10.1016/j.jphotobiol.2015.09.014.
- Palanisamy Iyapparaj; Peranandam Revathi; Ramasamy Ramasubburayan; Santhiyagu Prakash; Arunachalam Palavesam; Grasian Immanuel; Perumal Anantharaman; Asmita Sautreau; Claire Hellio; Antifouling and toxic properties of the bioactive metabolites from the seagrasses Syringodium isoetifolium and Cymodocea serrulata. Ecotoxicology and Environmental Safety 2014, 103, 54-60, 10.1016/j.ecoenv.2014.02.009.
- P. Iyapparaj; P. Revathi; R. Ramasubburayan; S. Prakash; Perumal Anantharaman; G. Immanuel; A. Palavesam; Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicology and Environmental Safety 2013, 89, 231-238, 10.1016/j.ecoenv.2012.12.001.
- Ravikumar, U; Vinoth, R; Selvan GP; Bioactive potential of a Seagrass Syringodium isoetifolium against bacterial fish pathogens.. J Pharm Res 2011, 4, 1854-1856, .
- Juwita, AP; Yamlaen, PVY; Edy, Hj; Formulasi Krim Ekstrak Etanol Daun Lamun (Syringodium isoetifolium). Pharmacon 2013, 2, 8-13, .
- Karthikeyan R; Sundarapandian M; Antidiabetic Activity of Methanolic Extract of Halodule uninervis in Streptozotocin-Induced Diabetic Mice. J Pharm Sci Res 2017, 9, 1864-1868, .
- Jazem A. Mahyoub; Al Thabiani Aziz; Chellasamy Panneerselvam; Kadarkarai Murugan; Mathath Roni; Subrata Trivedi; Marcello Nicoletti; Usama W. Hawas; Fekri M. Shaher; Muneer A. Bamakhrama; et al. Seagrasses as Sources of Mosquito Nano-Larvicides? Toxicity and Uptake of Halodule uninervis-Biofabricated Silver Nanoparticles in Dengue and Zika Virus Vector Aedes aegypti. Journal of Cluster Science 2016, 28, 565-580, 10.1007/s10876-016-1127-3.
- Karthikeyan, R. and Sundarapandian, M. 2017. Antidiabetic Activity of Methanolic Extract of Halodule uninervis in Streptozotocin-Induced Diabetic Mice. Journal of Pharmaceutical Sciences and Research. 9(10): 1864–1868.
- Nathan M. Gavin; Michael J. Durako; Localization and antioxidant capacity of flavonoids from intertidal and subtidal Halophila johnsonii and Halophila decipiens. Aquatic Botany 2011, 95, 242-247, 10.1016/j.aquabot.2011.07.004.
- Citra S.U. Dewi; Dedi Soedharma; Mujizat Kawaroe; KOMPONEN FITOKIMIA DAN TOKSISITAS SENYAWA BIOAKTIF DARI LAMUN ENHALUS ACOROIDES DAN THALASSIA HEMPRICHII DARI PULAU PRAMUKA, DKI JAKARTA. Jurnal Teknologi Perikanan dan Kelautan 2017, 3, 23, 10.24319/jtpk.3.23-27.
- Dewi, C. S. U., Soedharma, D. &Kawaroe, M. 2017. Komponen Fitokimia dan Toksisitas Senyawa Bioaktif dari Lamun Enhalus acoroides dan Thalassia Hemprichii danri Pulau Pramuka, DKI Jakarta. Jurnal Teknologi Perikanan dan Kelautan. 3(2): 23–27
- Septiani Septiani; Eko Nurcahya Dewi; Ima Wijayanti; AKTIVITAS ANTIBAKTERI EKSTRAK LAMUN (Cymodocea rotundata) TERHADAP BAKTERI Staphylococcus aureus DAN Escherichia coli (Antibacterial Activities of Seagrass Extracts (Cymodocea rotundata) Against Staphylococcus aureus and Escherichia coli). Saintek Perikanan : Indonesian Journal of Fisheries Science and Technology 2017, 13, 1-6, 10.14710/ijfst.13.1.1-6.
- Amudha P, Vanitha V, Pushpa BN, Jayalakshmi, M. Mohanasundaram S. Phytochemical analysis and invitro antioxidant screening of sea grass-Enhalus acoroides. Int J Res Pharm Sci. 2017;8(2):251-258.
- Shu-Hua Qi; Si Zhang; Pei-Yuan Qian; Bin-Gui Wang; Antifeedant, antibacterial, and antilarval compounds from the South China Sea seagrass Enhalus acoroides. Botanica Marina 2008, 51, 441-447, 10.1515/bot.2008.054.
