Small cell lung carcinoma (SCLC) is an aggressive and difficult to treat cancer. Although immunohistochemistry is not mandatory for a SCLC diagnosis, it might be required, especially in small samples. Insulinoma-associated protein 1 (INSM1) is expressed in endocrine and nervous tissues during embryogenesis, generally absent in adults and re-expressed in SCLC and other neuroendocrine neoplasms. Its high specificity propelled its use as diagnostic biomarker and an attractive therapeutic target. INSM1 is a highly sensitive (75–100%) and specific (82–100%) neuroendocrine immunohistochemical marker for SCLC diagnosis. It can be used in histological and cytological samples. Although advantageous, its standalone use is currently not recommended. Studies correlating INSM1 expression and prognosis have disclosed contrasting results, although the expression seemed to entail a worse survival. Targeting INSM1 effectively suppressed SCLC growth either as a suicide gene therapy regulator or as an indirect target of molecular-targeted therapy. INSM1 represents a valuable biomarker for SCLC diagnosis that additionally offers vast opportunities for the development of new prognostic and therapeutic strategies.
- INSM1
- biomarker
- immunohistochemistry
- small cell lung carcinoma
- diagnosis
- prognosis
- therapy
1. Introduction
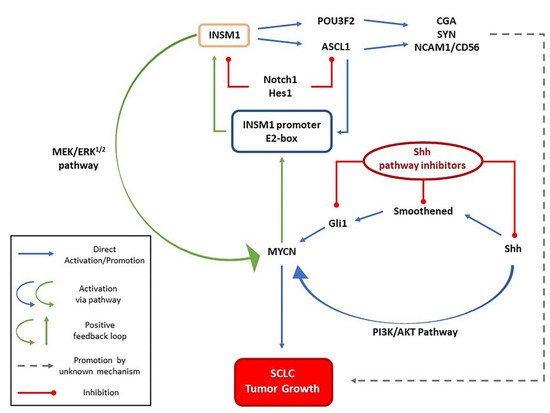
2. INSM1 and SCLC Oncogenesis
3. INSM1 Diagnostic Use
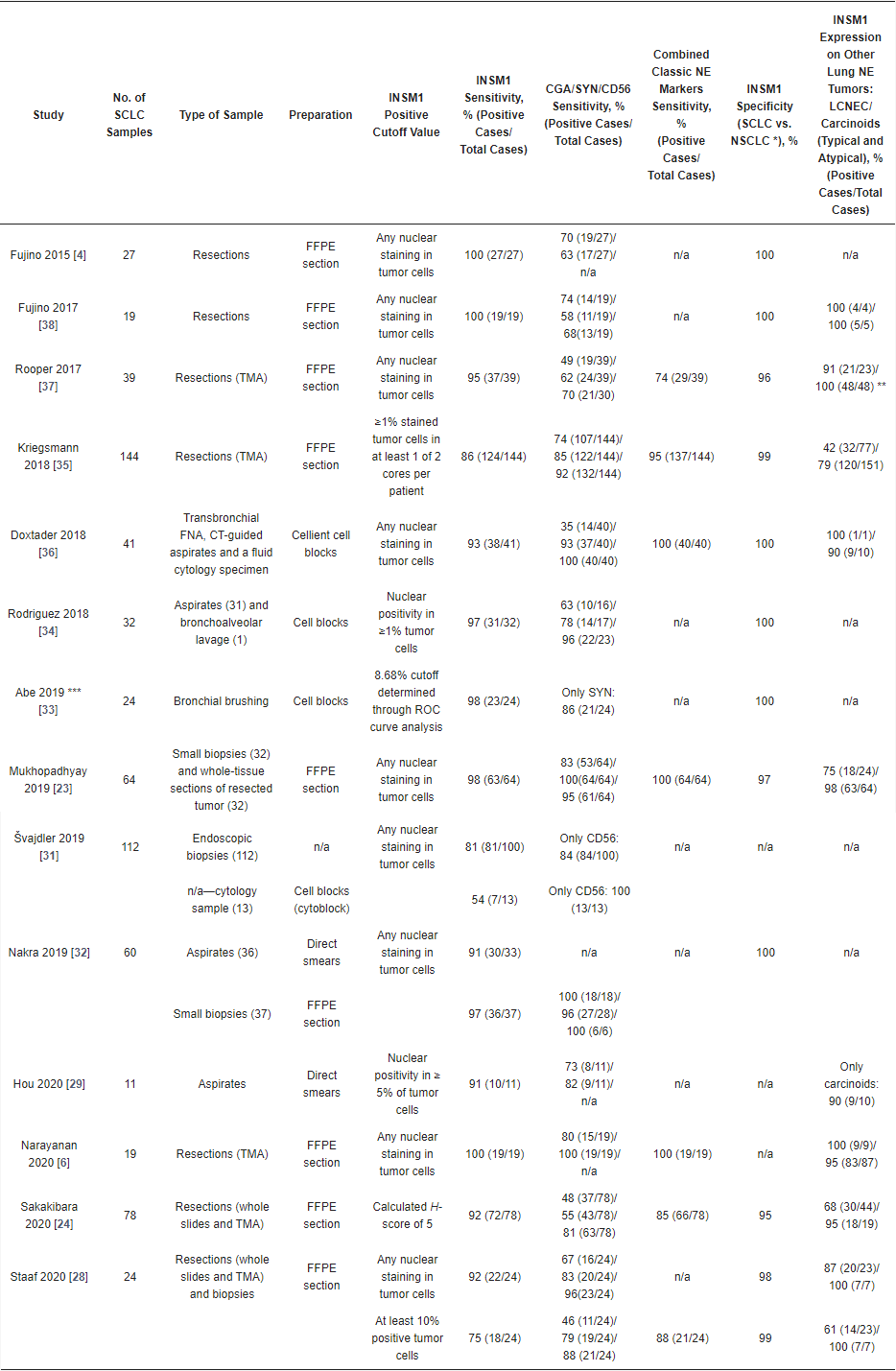
3.1. Immunohistochemistry
3.2. Immunocytochemistry
3.3. INSM1 Gene Expression and Diagnostic Use
3.4. INSM1 Diagnostic Use—Conclusions
4. INSM1 as a Prognostic Marker
5. Gene Therapy and Other Treatment Options Targeting INSM1 or Related Molecules
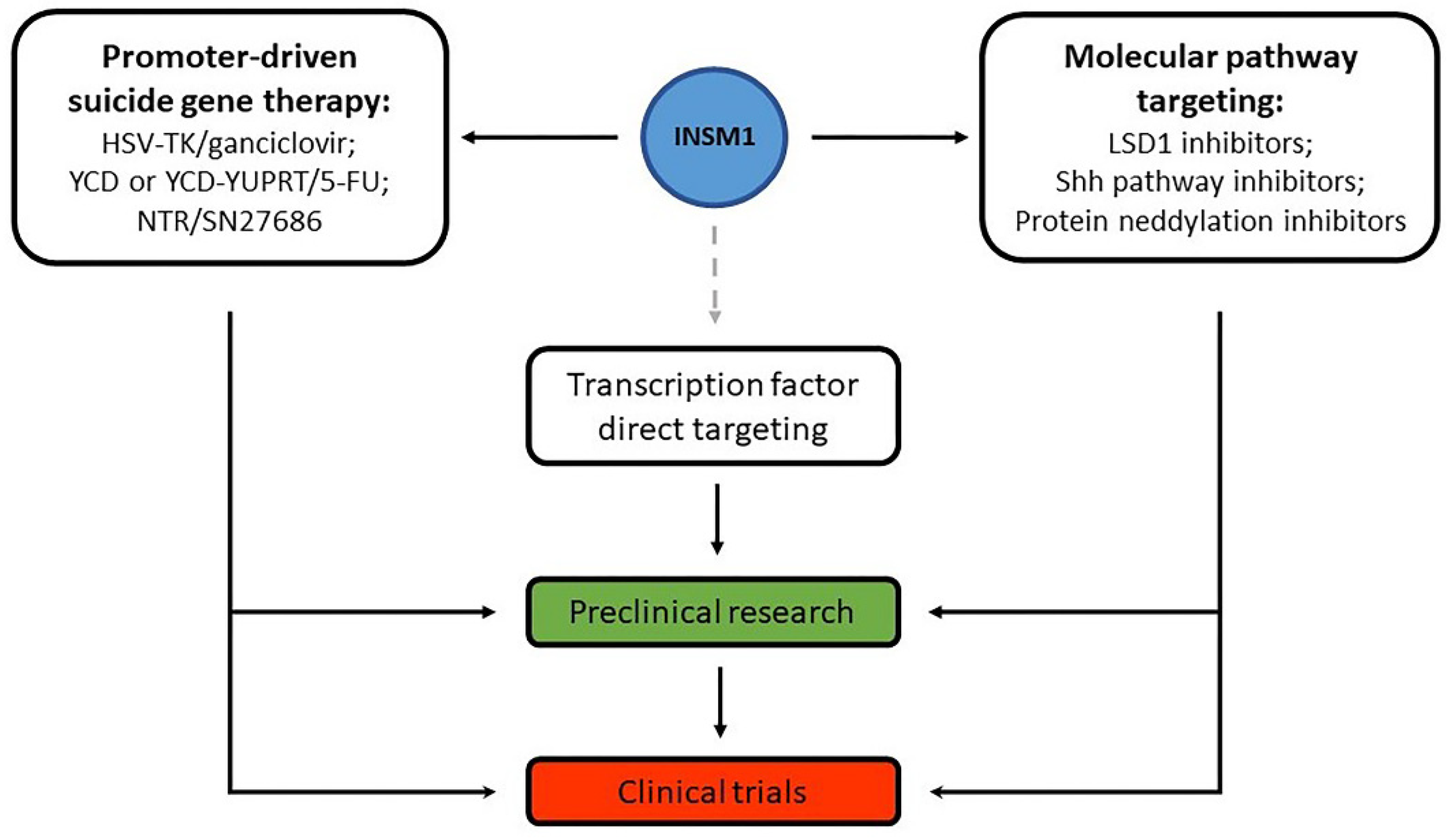
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jmp3030013
References
- Cancer.Net—Lung Cancer—Small Cell: Statistics. Available online: https://www.cancer.net/cancer-types/lung-cancer-small-cell/statistics (accessed on 18 April 2022).
- Rodriguez, E.; Lilenbaum, R.C. Small cell lung cancer: Past, present, and future. Curr. Oncol. Rep. 2010, 12, 327–334.
- Kris, M.G.; Benowitz, S.I.; Adams, S.; Diller, L.; Ganz, P.; Kahlenberg, M.S.; Le, Q.-T.; Markman, M.; Masters, G.A.; Newman, L.; et al. Clinical Cancer Advances 2010: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2010, 28, 5327–5347.
- Fujino, K.; Motooka, Y.; Hassan, W.A.; Ali Abdalla, M.O.; Sato, Y.; Kudoh, S.; Hasegawa, K.; Niimori-Kita, K.; Kobayashi, H.; Kubota, I.; et al. Insulinoma-Associated Protein 1 Is a Crucial Regulator of Neuroendocrine Differentiation in Lung Cancer. Am. J. Pathol. 2015, 185, 3164–3177.
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer Publications: Lyon, France, 2021; Volume 5.
- Narayanan, D.; Mandal, R.; Hardin, H.; Chanana, V.; Schwalbe, M.; Rosenbaum, J.; Buehler, D.; Lloyd, R.V. Long Non-coding RNAs in Pulmonary Neuroendocrine Neoplasms. Endocr. Pathol. 2020, 31, 254–263.
- Dingemans, A.M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 839–853.
- Thunnissen, E.; Borczuk, A.C.; Flieder, D.B.; Witte, B.; Beasley, M.B.; Chung, J.H.; Dacic, S.; Lantuejoul, S.; Russell, P.A.; den Bakker, M.; et al. The Use of Immunohistochemistry Improves the Diagnosis of Small Cell Lung Cancer and Its Differential Diagnosis. An International Reproducibility Study in a Demanding Set of Cases. J. Thorac. Oncol. 2017, 12, 334–346.
- Goto, Y.; De Silva, M.G.; Toscani, A.; Prabhakar, B.S.; Notkins, A.L.; Lan, M.S. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with "zinc-finger" DNA-binding motifs. J. Biol. Chem. 1992, 267, 15252–15257.
- Lan, M.S.; Breslin, M.B. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J. 2009, 23, 2024–2033.
- Gierl, M.S.; Karoulias, N.; Wende, H.; Strehle, M.; Birchmeier, C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 2006, 20, 2465–2478.
- Jia, S.; Wildner, H.; Birchmeier, C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev. Biol. 2015, 408, 90–98.
- Zombori, T.; Turkevi-Nagy, S.; Sejben, A.; Juhász-Nagy, G.; Cserni, G.; Furák, J.; Tiszlavicz, L.; Krenács, L.; Kővári, B. The panel of syntaxin 1 and insulinoma-associated protein 1 outperforms classic neuroendocrine markers in pulmonary neuroendocrine neoplasms. APMIS 2021, 129, 186–194.
- Zhu, J.; Wang, H.; Ramelot, T.A.; Kennedy, M.A.; Hu, R.; Yue, X.; Liu, M.; Yang, Y. Solution NMR structure of zinc finger 4 and 5 from human INSM1, an essential regulator of neuroendocrine differentiation. Proteins 2017, 85, 957–962.
- Osipovich, A.B.; Long, Q.; Manduchi, E.; Gangula, R.; Hipkens, S.B.; Schneider, J.; Okubo, T.; Stoeckert, C.J., Jr.; Takada, S.; Magnuson, M.A. Insm1 promotes endocrine cell differentiation by modulating the expression of a network of genes that includes Neurog3 and Ripply3. Development 2014, 141, 2939–2949.
- Breslin, M.B.; Zhu, M.; Notkins, A.L.; Lan, M.S. Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: Identification of consensus IA-1 binding sequence. Nucleic Acids Res. 2002, 30, 1038–1045.
- Xie, J.; Cai, T.; Zhang, H.; Lan, M.S.; Notkins, A.L. The zinc-finger transcription factor INSM1 is expressed during embryo development and interacts with the Cbl-associated protein. Genomics 2002, 80, 54–61.
- Lan, M.S.; Li, Q.; Lu, J.; Modi, W.S.; Notkins, A.L. Genomic organization, 5′-upstream sequence, and chromosomal localization of an insulinoma-associated intronless gene, IA-1. J. Biol. Chem. 1994, 269, 14170–14174.
- Tsai, H.K.; Hornick, J.L.; Vivero, M. INSM1 expression in a subset of thoracic malignancies and small round cell tumors: Rare potential pitfalls for small cell carcinoma. Mod. Pathol. 2020, 33, 1571–1580.
- Liu, W.D.; Wang, H.W.; Muguira, M.; Breslin, M.B.; Lan, M.S. INSM1 functions as a transcriptional repressor of the neuroD/beta2 gene through the recruitment of cyclin D1 and histone deacetylases. Biochem. J. 2006, 397, 169–177.
- Rosenbaum, J.N.; Guo, Z.; Baus, R.M.; Werner, H.; Rehrauer, W.M.; Lloyd, R.V. INSM1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. Am. J. Clin. Pathol. 2015, 144, 579–591.
- Rodriguez, E.F.; Fite, J.J.; Chowsilpa, S.; Maleki, Z. Insulinoma-associated protein 1 immunostaining on cytology specimens: An institutional experience. Hum. Pathol. 2019, 85, 128–135.
- Mukhopadhyay, S.; Dermawan, J.K.; Lanigan, C.P.; Farver, C.F. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: An immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod. Pathol. 2019, 32, 100–109.
- Sakakibara, R.; Kobayashi, M.; Takahashi, N.; Inamura, K.; Ninomiya, H.; Wakejima, R.; Kitazono, S.; Yanagitani, N.; Horiike, A.; Ichinose, J.; et al. Insulinoma-associated Protein 1 (INSM1) Is a Better Marker for the Diagnosis and Prognosis Estimation of Small Cell Lung Carcinoma Than Neuroendocrine Phenotype Markers Such as Chromogranin A, Synaptophysin, and CD56. Am. J. Surg. Pathol. 2020, 44, 757–764.
- Chen, C.; Breslin, M.B.; Lan, M.S. Ectopic expression of a small cell lung cancer transcription factor, INSM1 impairs alveologenesis in lung development. BMC Pulm. Med. 2016, 16, 49.
- Yu, L.; Dong, Y.; Xue, J.; Xu, S.; Wang, G.; Kuang, D.; Duan, Y. SOX11 is a sensitive and specific marker for pulmonary high-grade neuroendocrine tumors. Diagn. Pathol. 2022, 17, 2.
- Wang, M.; Abi-Raad, R.; Baldassarri, R.; Adeniran, A.J.; Cai, G. Expression of insulinoma-associated protein 1 in non-small cell lung cancers: A diagnostic pitfall for neuroendocrine tumors. Hum. Pathol. 2021, 115, 104–111.
- Staaf, J.; Tran, L.; Söderlund, L.; Nodin, B.; Jirström, K.; Vidarsdottir, H.; Planck, M.; Mattsson, J.S.M.; Botling, J.; Micke, P.; et al. Diagnostic Value of Insulinoma-Associated Protein 1 (INSM1) and Comparison With Established Neuroendocrine Markers in Pulmonary Cancers. Arch. Pathol. Lab. Med. 2020, 144, 1075–1085.
- Hou, T.; Gan, Q.; Joseph, C.T.; Sun, X.; Gong, Y. Insulinoma-associated protein 1 immunostaining for various types of neuroendocrine tumors on FNA smears. Cancer Cytopathol. 2020, 128, 725–732.
- Viswanathan, K.; Siddiqui, M.T.; Borczuk, A.C. Insulinoma-associated protein 1 is a sensitive and specific marker for lung neuroendocrine tumors in cytologic and surgical specimens. J. Am. Soc. Cytopathol. 2019, 8, 299–308.
- Švajdler, M.; Mezencev, R.; Šašková, B.; Ondič, O.; Mukenšnábl, P.; Michal, M. Triple marker composed of p16, CD56, and TTF1 shows higher sensitivity than INSM1 for diagnosis of pulmonary small cell carcinoma: Proposal for a rational immunohistochemical algorithm for diagnosis of small cell carcinoma in small biopsy and cytology specimens. Hum. Pathol. 2019, 85, 58–64.
- Nakra, T.; Nambirajan, A.; Guleria, P.; Phulware, R.H.; Jain, D. Insulinoma-associated protein 1 is a robust nuclear immunostain for the diagnosis of small cell lung carcinoma in cytology smears. Cancer Cytopathol. 2019, 127, 539–548.
- Abe, H.; Takase, Y.; Sadashima, E.; Fukumitsu, C.; Murata, K.; Ito, T.; Kawahara, A.; Naito, Y.; Akiba, J. Insulinoma-associated protein 1 is a novel diagnostic marker of small cell lung cancer in bronchial brushing and cell block cytology from pleural effusions: Validity and reliability with cutoff value. Cancer Cytopathol. 2019, 127, 598–605.
- Rodriguez, E.F.; Chowsilpa, S.; Maleki, Z. Insulinoma-Associated Protein 1 Immunostain: A Diagnostic Tool for Pulmonary Small Cell Carcinoma in Cytology. Acta Cytol. 2018, 62, 333–338.
- Kriegsmann, K.; Zgorzelski, C.; Kazdal, D.; Cremer, M.; Muley, T.; Winter, H.; Longuespée, R.; Kriegsmann, J.; Warth, A.; Kriegsmann, M. Insulinoma-associated Protein 1 (INSM1) in Thoracic Tumors is Less Sensitive but More Specific Compared With Synaptophysin, Chromogranin A, and CD56. Appl. Immunohistochem. Mol. Morphol. 2018, 28, 237–242.
- Doxtader, E.E.; Mukhopadhyay, S. Insulinoma-associated protein 1 is a sensitive and specific marker of neuroendocrine lung neoplasms in cytology specimens. Cancer Cytopathol. 2018, 126, 243–252.
- Rooper, L.M.; Sharma, R.; Li, Q.K.; Illei, P.B.; Westra, W.H. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am. J. Surg. Pathol. 2017, 41, 1561–1569.
- Fujino, K.; Yasufuku, K.; Kudoh, S.; Motooka, Y.; Sato, Y.; Wakimoto, J.; Kubota, I.; Suzuki, M.; Ito, T. INSM1 is the best marker for the diagnosis of neuroendocrine tumors: Comparison with CGA, SYP and CD56. Int. J. Clin. Exp. Pathol. 2017, 10, 5393–5405.
- Lan, M.S.; Russell, E.K.; Lu, J.; Johnson, B.E.; Notkins, A.L. IA-1, a new marker for neuroendocrine differentiation in human lung cancer cell lines. Cancer Res. 1993, 53, 4169–4171.
- Amelung, J.T.; Bührens, R.; Beshay, M.; Reymond, M.A. Key genes in lung cancer translational research: A meta-analysis. Pathobiology 2010, 77, 53–63.
- Tanigawa, M.; Nakayama, M.; Taira, T.; Hattori, S.; Mihara, Y.; Kondo, R.; Kusano, H.; Nakamura, K.; Abe, Y.; Ishida, Y.; et al. Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Med. Mol. Morphol. 2018, 51, 32–40.
- González, I.; Lu, H.C.; Sninsky, J.; Yang, C.; Bishnupuri, K.; Dieckgraefe, B.; Cao, D.; Chatterjee, D. Insulinoma-associated protein 1 expression in primary and metastatic neuroendocrine neoplasms of the gastrointestinal and pancreaticobiliary tracts. Histopathology 2019, 75, 568–577.
- McHugh, K.E.; Mukhopadhyay, S.; Doxtader, E.E.; Lanigan, C.; Allende, D.S. INSM1 Is a Highly Specific Marker of Neuroendocrine Differentiation in Primary Neoplasms of the Gastrointestinal Tract, Appendix, and Pancreas. Am. J. Clin. Pathol. 2020, 153, 811–820.
- Chen, J.F.; Yang, C.; Sun, Y.; Cao, D. Expression of novel neuroendocrine marker insulinoma-associated protein 1 (INSM1) in genitourinary high-grade neuroendocrine carcinomas: An immunohistochemical study with specificity analysis and comparison to chromogranin, synaptophysin, and CD56. Pathol.-Res. Pract. 2020, 216, 152993.
- Kim, I.E., Jr.; Amin, A.; Wang, L.J.; Cheng, L.; Perrino, C.M. Insulinoma-associated Protein 1 (INSM1) Expression in Small Cell Neuroendocrine Carcinoma of the Urinary Tract. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 687–693.
- Xin, Z.; Zhang, Y.; Jiang, Z.; Zhao, L.; Fan, L.; Wang, Y.; Xie, S.; Shangguan, X.; Zhu, Y.; Pan, J.; et al. Insulinoma-associated protein 1 is a novel sensitive and specific marker for small cell carcinoma of the prostate. Hum. Pathol. 2018, 79, 151–159.
- Kuji, S.; Watanabe, R.; Sato, Y.; Iwata, T.; Hirashima, Y.; Takekuma, M.; Ito, I.; Abe, M.; Nagashio, R.; Omae, K.; et al. A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: Analysis of 37 cases. Gynecol. Oncol. 2017, 144, 384–390.
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. INSM1 Is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am. J. Surg. Pathol. 2018, 42, 665–671.
- Yuan, C.; Jiao, F.; Zhai, C.; Zhang, J.; Wang, S.; Zhu, L. Application of INSM1 in Diagnosis and Grading of Laryngeal Neuroendocrine Carcinoma. Laryngoscope 2021, 131, E2662–E2668.
- Parra, O.; Linos, K.; Yan, S.; Lilo, M.; LeBlanc, R.E. Comparative performance of insulinoma-associated protein 1 (INSM1) and routine immunohistochemical markers of neuroendocrine differentiation in the diagnosis of endocrine mucin-producing sweat gland carcinoma. J. Cutan. Pathol. 2021, 48, 41–46.
- Rush, P.S.; Rosenbaum, J.N.; Roy, M.; Baus, R.M.; Bennett, D.D.; Lloyd, R.V. Insulinoma-associated 1: A novel nuclear marker in Merkel cell carcinoma (cutaneous neuroendocrine carcinoma). J. Cutan. Pathol. 2018, 45, 129–135.
- Lilo, M.T.; Chen, Y.; LeBlanc, R.E. INSM1 Is More Sensitive and Interpretable than Conventional Immunohistochemical Stains Used to Diagnose Merkel Cell Carcinoma. Am. J. Surg. Pathol. 2018, 42, 1541–1548.
- Maleki, Z.; Abram, M.; Dell’Aquila, M.; Kilic, I.; Lu, R.; Musarra, T.; Barkan, G.; Rajakorpi, E.; Rossi, E.D.; Kholová, I. Insulinoma-associated protein 1 (INSM-1) expression in medullary thyroid carcinoma FNA: A multi-institutional study. J. Am. Soc. Cytopathol. 2020, 9, 185–190.
- Yoshida, A.; Makise, N.; Wakai, S.; Kawai, A.; Hiraoka, N. INSM1 expression and its diagnostic significance in extraskeletal myxoid chondrosarcoma. Mod. Pathol. 2018, 31, 744–752.
- Wang, H.; Krishnan, C.; Charville, G.W. INSM1 Expression in Peripheral Neuroblastic Tumors and Other Embryonal Neoplasms. Pediatr. Dev. Pathol. 2019, 22, 440–448.
- Farkas, L.M.; Haffner, C.; Giger, T.; Khaitovich, P.; Nowick, K.; Birchmeier, C.; Pääbo, S.; Huttner, W.B. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron 2008, 60, 40–55.
- Wildner, H.; Gierl, M.S.; Strehle, M.; Pla, P.; Birchmeier, C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development 2008, 135, 473–481.
- Mahalakshmi, B.; Baskaran, R.; Shanmugavadivu, M.; Nguyen, N.T.; Velmurugan, B.K. Insulinoma-associated protein 1 (INSM1): A potential biomarker and therapeutic target for neuroendocrine tumors. Cell. Oncol. 2020, 43, 367–376.
- Chen, C.; Notkins, A.L.; Lan, M.S. Insulinoma-Associated-1: From Neuroendocrine Tumor Marker to Cancer Therapeutics. Mol. Cancer Res. 2019, 17, 1597–1604.
- Zhang, T.; Chen, C.; Breslin, M.B.; Song, K.; Lan, M.S. Extra-nuclear activity of INSM1 transcription factor enhances insulin receptor signaling pathway and Nkx6.1 expression through RACK1 interaction. Cell. Signal. 2014, 26, 740–747.
- Duggan, A.; Madathany, T.; de Castro, S.C.; Gerrelli, D.; Guddati, K.; García-Añoveros, J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J. Comp. Neurol. 2008, 507, 1497–1520.
- Pedersen, N.; Pedersen, M.W.; Lan, M.S.; Breslin, M.B.; Poulsen, H.S. The insulinoma-associated 1: A novel promoter for targeted cancer gene therapy for small-cell lung cancer. Cancer Gene Ther. 2006, 13, 375–384.
- Wang, H.W.; Breslin, M.B.; Chen, C.; Akerstrom, V.; Zhong, Q.; Lan, M.S. INSM1 promoter-driven adenoviral herpes simplex virus thymidine kinase cancer gene therapy for the treatment of primitive neuroectodermal tumors. Hum. Gene Ther. 2009, 20, 1308–1318.
- Christensen, C.L.; Gjetting, T.; Poulsen, T.T.; Cramer, F.; Roth, J.A.; Poulsen, H.S. Targeted cytosine deaminase-uracil phosphoribosyl transferase suicide gene therapy induces small cell lung cancer-specific cytotoxicity and tumor growth delay. Clin. Cancer Res. 2010, 16, 2308–2319.
- Michaelsen, S.R.; Christensen, C.L.; Sehested, M.; Cramer, F.; Poulsen, T.T.; Patterson, A.V.; Poulsen, H.S. Single agent- and combination treatment with two targeted suicide gene therapy systems is effective in chemoresistant small cell lung cancer cells. J. Gene Med. 2012, 14, 445–458.
- Akerstrom, V.; Chen, C.; Lan, M.S.; Breslin, M.B. Modifications to the INSM1 promoter to preserve specificity and activity for use in adenoviral gene therapy of neuroendocrine carcinomas. Cancer Gene Ther. 2012, 19, 828–838.
- Akerstrom, V.; Chen, C.; Lan, M.S.; Breslin, M.B. Adenoviral insulinoma-associated protein 1 promoter-driven suicide gene therapy with enhanced selectivity for treatment of neuroendocrine cancers. Ochsner J. 2013, 13, 91–99.
- Tseng, A.W.; Chen, C.; Breslin, M.B.; Lan, M.S. Tumor-specific promoter-driven adenoviral therapy for insulinoma. Cell. Oncol. 2016, 39, 279–286.
- Welcker, J.E.; Hernandez-Miranda, L.R.; Paul, F.E.; Jia, S.; Ivanov, A.; Selbach, M.; Birchmeier, C. Insm1 controls development of pituitary endocrine cells and requires a SNAG domain for function and for recruitment of histone-modifying factors. Development 2013, 140, 4947–4958.
- Chen, C.; Breslin, M.B.; Lan, M.S. INSM1 increases N-myc stability and oncogenesis via a positive-feedback loop in neuroblastoma. Oncotarget 2015, 6, 36700–36712.
- Tao, W.; Zhang, Y.; Ma, L.; Deng, C.; Duan, H.; Liang, X.; Liao, R.; Lin, S.; Nie, T.; Chen, W.; et al. Haploinsufficiency of Insm1 Impairs Postnatal Baseline β-Cell Mass. Diabetes 2018, 67, 2615–2625.
- Morimoto, M.; Nishinakamura, R.; Saga, Y.; Kopan, R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012, 139, 4365–4373.
- Peake, J.L.; Reynolds, S.D.; Stripp, B.R.; Stephens, K.E.; Pinkerton, K.E. Alteration of pulmonary neuroendocrine cells during epithelial repair of naphthalene-induced airway injury. Am. J. Pathol. 2000, 156, 279–286.
- Taniwaki, M.; Daigo, Y.; Ishikawa, N.; Takano, A.; Tsunoda, T.; Yasui, W.; Inai, K.; Kohno, N.; Nakamura, Y. Gene expression profiles of small-cell lung cancers: Molecular signatures of lung cancer. Int. J. Oncol. 2006, 29, 567–575.
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3.
- Kudoh, S.; Tenjin, Y.; Kameyama, H.; Ichimura, T.; Yamada, T.; Matsuo, A.; Kudo, N.; Sato, Y.; Ito, T. Significance of achaete-scute complex homologue 1 (ASCL1) in pulmonary neuroendocrine carcinomas; RNA sequence analyses using small cell lung cancer cells and Ascl1-induced pulmonary neuroendocrine carcinoma cells. Histochem. Cell Biol. 2020, 153, 443–456.
- Chen, C.; Breslin, M.B.; Lan, M.S. Sonic hedgehog signaling pathway promotes INSM1 transcription factor in neuroendocrine lung cancer. Cell. Signal. 2018, 46, 83–91.
- Hamanaka, W.; Motoi, N.; Ishikawa, S.; Ushijima, M.; Inamura, K.; Hatano, S.; Uehara, H.; Okumura, S.; Nakagawa, K.; Nishio, M.; et al. A subset of small cell lung cancer with low neuroendocrine expression and good prognosis: A comparison study of surgical and inoperable cases with biopsy. Hum. Pathol. 2014, 45, 1045–1056.
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E.; Hasleton, P.S.; Colby, T.V.; Sheppard, M.N.; Falk, R.; Travis, W.D. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197.
- Nandeesh, B.; Crasta, J.; Tirumalae, R. Fine-needle aspiration cytology in the diagnosis and typing of lung carcinomas. Clin. Cancer Investig. J. 2015, 4, 637–644.
- Roy-Chowdhuri, S.; Aisner, D.L.; Allen, T.C.; Beasley, M.B.; Borczuk, A.; Cagle, P.T.; Capelozzi, V.; Dacic, S.; da Cunha Santos, G.; Hariri, L.P.; et al. Biomarker Testing in Lung Carcinoma Cytology Specimens: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2016, 140, 1267–1272.
- Zhao, L.; Guo, M.; Sneige, N.; Gong, Y. Value of PAX8 and WT1 Immunostaining in Confirming the Ovarian Origin of Metastatic Carcinoma in Serous Effusion Specimens. Am. J. Clin. Pathol. 2012, 137, 304–309.
- Gong, Y.; Symmans, W.F.; Krishnamurthy, S.; Patel, S.; Sneige, N. Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer 2004, 102, 34–40.
- Eichhorn, F.; Dienemann, H.; Muley, T.; Warth, A.; Hoffmann, H. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann. Thorac. Surg. 2015, 99, 983–989.
- Tanaka, Y.; Ogawa, H.; Uchino, K.; Ohbayashi, C.; Maniwa, Y.; Nishio, W.; Nakao, A.; Yoshimura, M. Immunohistochemical studies of pulmonary large cell neuroendocrine carcinoma: A possible association between staining patterns with neuroendocrine markers and tumor response to chemotherapy. J. Thorac. Cardiovasc. Surg. 2013, 145, 839–846.
- Xu, X.; Wang, G.; Duan, Y.; Huo, Z. Prognostic value and non-neuroendocrine role of INSM1 in small cell lung cancer. Pathol.-Res. Pract. 2021, 229, 153693.
- Minami, K.; Jimbo, N.; Tanaka, Y.; Ogawa, H.; Hokka, D.; Nishio, W.; Yoshimura, M.; Itoh, T.; Maniwa, Y. Insulinoma-associated protein 1 is a prognostic biomarker in pulmonary high-grade neuroendocrine carcinoma. J. Surg. Oncol. 2020, 122, 243–253.
- Baine, M.K.; Hsieh, M.S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. 2020, 15, 1823–1835.
- McColl, K.; Wildey, G.; Sakre, N.; Lipka, M.B.; Behtaj, M.; Kresak, A.; Chen, Y.; Yang, M.; Velcheti, V.; Fu, P.; et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 2017, 8, 73745–73756.
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012, 2, 798–811.
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297.
- Lee, Y.; Joo, J.; Lee, Y.J.; Lee, E.K.; Park, S.; Kim, T.S.; Lee, S.H.; Kim, S.Y.; Wie, G.A.; Park, M.; et al. Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer 2021, 151, 8–15.
- Fan, H.; Bai, S.; Guan, X.; Ma, W.; Fu, Y.; Zhang, X.; Deng, L.; Tian, J. Metformin improves survival in patients with concurrent diabetes and small cell lung cancer: A meta-analysis. Minerva Endocrinol. 2021, in press.
- Chun, S.G.; Liao, Z.; Jeter, M.D.; Chang, J.Y.; Lin, S.H.; Komaki, R.U.; Guerrero, T.M.; Mayo, R.C.; Korah, B.M.; Koshy, S.M.; et al. Metabolic Responses to Metformin in Inoperable Early-stage Non-Small Cell Lung Cancer Treated With Stereotactic Radiotherapy: Results of a Randomized Phase II Clinical Trial. Am. J. Clin. Oncol. 2020, 43, 231–235.
- Zeng, S.; Gan, H.X.; Xu, J.X.; Liu, J.Y. Metformin improves survival in lung cancer patients with type 2 diabetes mellitus: A meta-analysis. Med. Clin. 2019, 152, 291–297.
- Lu, H.; Xie, F.; Huang, Z.; Qin, J.; Han, N.; Mao, W. Effect of metformin in the prognosis of patients with small-cell lung cancer combined with diabetes mellitus. Adv. Clin. Exp. Med. 2018, 27, 1195–1199.
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359.
- Iams, W.T.; Porter, J.; Horn, L. Immunotherapeutic approaches for small-cell lung cancer. Nat. Rev. Clin. Oncol. 2020, 17, 300–312.
- Takagi, S.; Ishikawa, Y.; Mizutani, A.; Iwasaki, S.; Matsumoto, S.; Kamada, Y.; Nomura, T.; Nakamura, K. LSD1 Inhibitor T-3775440 Inhibits SCLC Cell Proliferation by Disrupting LSD1 Interactions with SNAG Domain Proteins INSM1 and GFI1B. Cancer Res. 2017, 77, 4652–4662.
- Norton, J.P.; Augert, A.; Eastwood, E.; Basom, R.; Rudin, C.M.; MacPherson, D. Protein neddylation as a therapeutic target in pulmonary and extrapulmonary small cell carcinomas. Genes Dev. 2021, 35, 870–887.
- Chen, C.; Breslin, M.B.; Guidry, J.J.; Lan, M.S. 5′-Iodotubercidin represses insulinoma-associated-1 expression, decreases cAMP levels, and suppresses human neuroblastoma cell growth. J. Biol. Chem. 2019, 294, 5456–5465.
- Breslin, M.B.; Zhu, M.; Lan, M.S. NeuroD1/E47 regulates the E-box element of a novel zinc finger transcription factor, IA-1, in developing nervous system. J. Biol. Chem. 2003, 278, 38991–38997.
- Tseng, A.W.; Akerstrom, V.; Chen, C.; Breslin, M.B.; Lan, M.S. Detection of neuroendocrine tumors using promoter-specific secreted Gaussia luciferase. Int. J. Oncol. 2016, 48, 173–180.
