Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Mercury is a toxic pollutant that can negatively impact the population’s health and the environment. The research on atmospheric mercury is of critical concern because of the diverse process that this pollutant suffers in the atmosphere as well as its deposition capacity, which can provoke diverse health issues.
- atmospheric mercury
- Minamata Convention
- southern hemisphere
1. Introduction
The increments in the concentration of atmospheric pollutants have generated problems for people’s health and the environment [1]. One toxic pollutant that can harm the humans is mercury. In addition, low concentrations of this metal can severely damage people’s health and lead to conditions such as cardiovascular or reproductive illnesses. However, the adverse effects of this metal depend on diverse factors such as exposure or chemical composition; therefore, to investigate the mercury cycle, current and future levels are relevant [2]. Although mercury in the atmosphere comes from natural processes such as volcanic activity, anthropogenic activities, such as stationary coal combustion or the gold mining industry, are the main sources of mercury release into the environment and disrupt the natural cycle of mercury [2]. Although mercury can be present in the air, water, and on the ground, a large amount of anthropogenic mercury pollution reaches the atmosphere and enters ecosystems through dry and wet deposition. The study of mercury has become relevant in the last 30 years and there have been diverse scientific events related to mercury since 1990 [3]. However, one of the main initiatives that encourages the protection of the environment and the population’s health is the Minamata Convention on Mercury. This convention started in 2017 and was implemented based on scientific reports from the International Conference on Mercury as a Global Pollutant (ICMGP) [3]. Between the 35 articles of the convention, the scientific contribution is crucial for the success of their goals. To illustrate, the articles of the Minamata Convention include mercury emissions in air, reinforcing mercury monitoring systems, providing mercury data baselines, and enhancing modelling techniques, among others [3][4].
One of the main adverse issues of mercury is the bioaccumulation that it can have. Although atmospheric mercury can deposit on land and water, the surfaces remit part of it into the atmosphere [4]. However, another percentage is methylated and deposited into the ecosystem. Indeed, inorganic mercury can be methylated and bioaccumulated in aquatic environments and biomagnified in the aquatic food chain [5][6]. In this context, although found in several types of foods, researchers measured the levels of mercury; fish revealed higher levels of this mental. Fish are one of the most crucial exposure routes for humans [2][5]. Fish bioaccumulate methylmercury in their muscle tissues; however, higher levels of mercury were found in some fish species such as swordfish, tilefish, and sharks. Therefore, people with a diet based on fish and marine mammals could have greater exposure to mercury [2].
Mercury in the atmosphere can be in diverse phases, such as gas and solid particles (clouds and aerosols). In the atmosphere, this element can be found as gaseous oxidized mercury (GOM), Hg2, particulate-bound mercury (PBM), and gaseous elemental mercury (GEM), Hg0 [6][7][8][9]. GEM is the more abundant form in the atmosphere, which is relatively inert. Indeed, it can travel long distances before it is modified with oxidation chemical reactions in the atmosphere or removed by some receptors such as those in plants [5]. In contrast, the fastest deposition occurs for oxidized mercury compounds because of their greater solubility in water. For this reason, GOM and GEM can negatively affect local ecosystems unlike elemental mercury, which is less reactive and has a long lifetime [10]. Although the pollutant can be found in three forms, there are several transformation phenomena in which mercury suffers as well as in transport. Besides oxidation and reduction reactions, complex formations occur and phases change, especially when there is an interaction between the atmosphere and the ocean, bioaccumulation, and between other phenomena [11].
Atmospheric modelling plays a crucial role in reaching the Minamata Convention aims. In this respect, meteorological and air quality modelling help to predict and forecast using current data, meteorological phenomena, and the behaviour of the concentration of various pollutants [12][13]. In particular, there is a lack of real-time studies of certain compounds, such as mercury, and models for predicting air quality and for simulating and studying diverse scenarios [12]. The equations that govern atmospheric modelling consider several parameters such as pressure, temperature, speed, and concentration, among others. Hence, developing a model requires supercomputers to perform the simulations for a precise and detailed prediction because of the spatial variations in the parameters [12][13]. Various meteorological and air quality models have been used to study different pollutants including mercury. However, for atmospheric mercury, most of the investigations are on a global scale or in the Northern Hemisphere (NH) [5][8][14][15][16][17]. The main reason for this is the information available. There are several places measuring mercury in the NH in contrast to the Southern Hemisphere (SH) where there is a deficient number [8][14][18]. In fact, in the SH, there are six monitoring sites, of which only one in Australia measures the wet deposition of mercury. In contrast, in the NH, 123 sites measure wet deposition [14]. This situation increases the complexity of comparing the results of simulations with observed data.
Chemical transport models (CTM) can study how the atmosphere is affected by the pollutants emitted, mathematically exposing diverse phenomena such as transport caused by wind and dispersion caused by turbulent movements, among others [19]. For mercury, CTM are highly relevant because of the lack of real-time measurements of this compound and the problems it can cause for people’s health [5]. In recent decades, atmospheric models have been developed analysing mercury, considering O3, OH radicals, and halogen species as the primary oxidants of Hg0. However, there is still a discrepancy about which oxidant is dominant [20]. The main pathway oxidations used for Hg0 are OH/O3 and Br where adequate results have been obtained. However, mercury chemistry, for which there are multiple Hg0 oxidants, is more complex [20]. Using CTMs in mercury atmospheric predictions has some advantages. For instance, it is possible to evaluate potential policy outcomes to estimate and analyse the changes in relevant factors such as mercury deposition. These models also allow the evaluation of proposed measures to determine their potential efficacy in mercury abatement or worldwide reduction [21]. Further, CTMs can give wide regional and seasonal variations in Hg0 concentrations to compensate for the absence of real-time observed data. Accurate global estimates of mercury dispersion can be obtained by reproducing the time and spatial patterns of mercury measurements [21][22]. However, CTMs also have some disadvantages. Indeed, when different atmospheric redox mechanisms are included in the models, recent investigations have shown that it is still difficult to reproduce the patterns of the observed concentrations [21]. One area where uncertainty persists is the potential importance of heterogeneous chemistry, an aspect that models have been unsuccessful at incorporating. The uncertainty of emissions inventories is another problem with CTMs. The dependence on inventory data, particularly the amount of mercury released from natural sources, has become a significant obstacle in studies of atmospheric models [21][22][23].
2. Emissions of Mercury
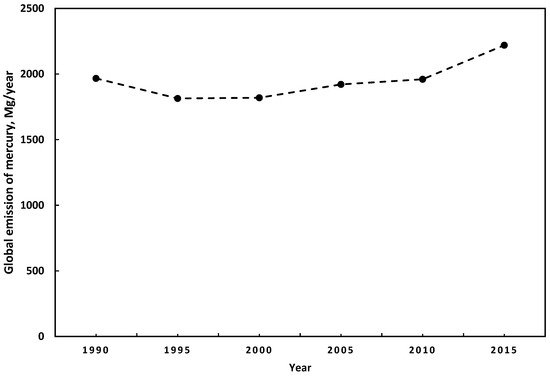
Estimates of anthropogenic mercury emissions have a particular uncertainty. The principal reasons are the lack of precision in the estimation methods, the low participation of some countries in the estimation of emissions, and the lack of consideration of specific activities in the estimation of mercury’s emissions inventory [9]. However, some studies suggest a consensus regarding the main sources of anthropogenic mercury emissions. In 2015, the highest emissions were from small-scale gold mining, followed by the combustion of fuels for different uses, and industry sectors. Further, compared to the global mercury emissions of anthropogenic sources from 2010 to 2015, they increased by 410 Mg/year [21][24]. China is the country with the highest level of mercury emissions [24]. Figure 1 presents the trends in global mercury emissions with anthropogenic origins from 1990 to 2015. During 2010 and 2015, there were no significant changes in the emissions patterns. The most significant emissions come from Asia for both years, representing around 48.8%, followed by South America with 18.4% and 16.2% from sub-Saharan Africa. The region with the most significant anthropogenic mercury emissions is East and Southeast Asia with 859 Mg/year, representing 38.6% of the total. In addition, regarding fixed emissions sources, fossil fuel combustion and biomass burning represent around 24% of global mercury emissions [21].
The global emissions inventory of mercury into the atmosphere from anthropogenic sources is estimated to be close to 2220 Mg/year [21]. Gold mining, including artisanal and small-scale, is responsible for around 838 Mg/year, followed by diverse industrial sectors with 613.6 Mg/year, and the combustion of fuels to produce energy for different uses is responsible for 481 Mg/year [21]. Table 1 shows the details of the three sectors and the various subdivisions and sources of each sector. This table illustrates that 99.53% (2189.7 Mg/year) of the total mercury emissions come from anthropogenic sources (1932.6 Mg/year). In the SH, around 68% of the anthropogenic mercury emissions come from artisanal and small-scale gold mining (ASGM). Also, most of the emissions come from the latitudes +30° to −30° [25]. Most of the emissions for ASGM are from South America, with 340 Mg/year, representing 40.6% of the total for this sector group, where Peru has the highest emissions with 110.4 Mg/year [21]. In addition, African countries are responsible for about 30.1% of the ASGM mercury emissions (252 Mg/year); however, the countries in the SH of the continent represent around 22% of the African contribution. Regarding Oceania, this continent does not register emissions for the ASGM sector. Further, some countries partly in the SH have high mercury emissions levels in the ASGM sector, such as Indonesia, with 124.5 Mg/year [21][26].
Table 1. Main sectors of anthropogenic mercury emissions (A) and natural emissions (N). The anthropogenic emissions to the atmosphere are for 2015 and based on 2200 Mg/year and the table shows 99.53% of the total anthropogenic emissions [21]. The natural emissions are for 2008 and based on 5207 Mg/year; adapted from Pirrone et al. (2010) [9][28][29].
| Source | Type | Sector | Emissions Mg/Year | Contribution % |
|---|---|---|---|---|
| Artisanal gold production | A | Artisanal and small-scale gold mining | 838 | 11.31% |
| Combustion of fuels (energy, industry, and domestic/residential uses) | A | Stationary combustion of coal for transportation, domestic, and residential. | 55.8 | 0.75% |
| Stationary combustion of gas for transportation, domestic, and residential. | 0.165 | 0.00% | ||
| Stationary combustion of oil for transportation, domestic, and residential. | 2.7 | 0.04% | ||
| Industrial stationary combustion of coal. | 126 | 1.70% | ||
| Industrial stationary combustion of gas. | 0.123 | 0.002% | ||
| Industrial stationary combustion of oil. | 1.4 | 0.02% | ||
| Power plants’ stationary combustion of coal | 292 | 3.94% | ||
| Power plants’ stationary combustion of gas | 0.349 | 0.002% | ||
| Power plants’ stationary combustion of oil | 2.45 | 0.03% | ||
| Diverse industrial sectors | A | Raw materials and fuel for cement production (excluding coal) | 233 | 3.15% |
| Production of non-ferrous metals, including Al, Cu, Pb and Zn | 228 | 3.08% | ||
| Gold production on a large scale | 84.5 | 1.14% | ||
| Production of mercury | 13.8 | 0.19% | ||
| Refining of oil | 14.4 | 0.19% | ||
| Production of steel and pig iron (primary) | 29.8 | 0.40% | ||
| Secondary production of steel | 10.1 | 0.14% | ||
| Biomass burning | A | Biomass burning from domestic, industrial, and power plants | 51.9 | 0.70% |
| Waste | A | Other waste | 147 | 1.98% |
| Vinyl-chloride monomer | A | Vinyl-chloride monomer production (mercury catalyst) and vinyl-chloride monomer recycling | 58.2 | 0.79% |
| Oceans | N | Re-emissions from the ocean | 2682 | 36.21% |
| Biomass Burning | N | Emissions from accumulated mercury in biomass | 675 | 9.11% |
| Arid areas | N | Deserts and metalliferous and non-vegetated zones | 546 | 7.37% |
| Tundra/Grassland/Savannah | N | Tundra/Grassland/Savannah | 448 | 6.05% |
| Forests | N | Forests | 342 | 4.62% |
| Evasion after mercury depletion events | N | Evasion after mercury depletion events | 200 | 2.70% |
| Agricultural areas | N | Areas with agricultural processes | 128 | 1.73% |
| Lakes | N | Lakes | 96 | 1.30% |
| Geothermal activities | N | Volcanoes and geothermal activities | 90 | 1.22% |
The percentage contribution between anthropogenic and natural emissions depends on the model used to estimate the emissions. Indeed, the anthropogenic/natural ratio can be different for diverse models. For instance, using the GEOS-chem model, the ratio could be 0.583 and using the GRAHM model it could be 0.629 [9]. However, considering a natural emissions estimation for 2008, the emissions are about 5207 Mg/year [9][28][29]. Using the most recent estimation for anthropogenic emissions, which was 2200 Mg/year [21], the total mercury emissions could be around 7407 Mg/year, of which the natural contribution is around 70.30% providing an anthropogenic/natural emissions ratio of 0.423. However, there is uncertainty associated with the estimation of both anthropogenic and biogenic emissions. This is because of differences in emissions factors, uncertainties in emissions reports from some countries, and comparisons of emissions with field measurements [29]. With biogenic sources, it is necessary to consider two aspects. The mercury emissions emitted directly from natural processes are the primary sources such as volcanic emissions. Further, the secondary sources are the re-emissions of this metal because of a previously accumulated deposition on both marine and terrestrial surfaces such as biomass burning [28]. For the natural sources of mercury, Table 1 shows the details of the estimations for global emissions by 2008. In that case, the most significant contribution was made by remissions from the oceans with 2682 Mg/year, followed by biomass burning with 675 Mg/year, and deserts and metalliferous and non-vegetated zones with 546 Mg/year. Indeed, most of the natural emissions come from the remission process, representing a considerable proportion of the total mercury emissions. For instance, oceans and biomass burning from natural sources represent around 45.32% of the total mercury emissions inventory [28][29].
Regarding biomass burning mercury emissions (BBME) from natural sources, Africa is the most significant contributor, followed by Asia, and then the Americas with 41%, 31%, and 28%, respectively [30]. Indeed, there is also an essential contribution from the SH. Considering the annual emissions of 675 Mg of mercury, equatorial Asia has around 28.44%, followed by boreal Asia with 14.67%, and the southern part of South America with 14.07%. To summarise, about 53% of the BBME comes from the SH [31]. The vegetation plays a crucial role in the BBME because of forest fires. Shi et al. (2019) related the most significant amount of mercury emissions to forest fires from tropical forests [30]. Tropical forests are found to a greater extent in SH areas such as Bolivia, Central Africa, and the central region of Brazil, in addition to NH areas such as Laos, Cambodia, and Myanmar [30]. The second type of vegetation with a high level of BBME is woody savanna/shrubland. It is mainly found in Africa, concentrated in Central Africa and East Africa, in addition to Cambodia and Myanmar [30].
3. Mercury and Health Effects
Although the atmosphere is the most relevant transport route for mercury, the processes and phenomena that occur on land and in the oceans play a relevant role in its redistribution. Methylmercury (CH3Hg) becomes relevant as the principal route of human exposure is through the consumption of fish [32]. This neurotoxic contaminant can provoke specific adverse health effects such as cardiovascular or neurocognitive illnesses. For instance, through food consumption, which is the primary exposure route, a study in China attributed around 10,000 deaths per year because of heart attacks provoked by this metal [33]. The negative impact of mercury on humans depends on diverse factors such as chemical composition, exposure time, and concentration. Among the health issues are impaired neurological development, arrhythmias, cardiomyopathy, and kidney damage. In addition, high doses of mercury vapour inhalation can cause respiratory failure [2].
There are four broad categories into which it is possible to divide the health effects that mercury exposure can produce. These are neurological, renal, cardiovascular, and reproductive problems. Regarding the neurological effects, some studies have shown that prolonged exposure to mercury in low doses can cause sleep disorders, mood changes, and various neurological diseases [2][34]. For example, adverse effects have been found with levels of inorganic mercury in urine close to <4 µg/L [2]. Another neurological problem is memory loss because of the deactivation of enzymes required to produce energy in brain cells. In addition, studies have shown that mercury is neurotoxic, which is why it can cause significant damage to the brain and nerve cells [2][35].
Regarding the negative effects at the kidney level, mercury affects not only human beings but also other mammals because the kidneys accumulate mercury ions. Unlike organic mercury compounds, oral exposure to inorganic mercury salts at low and prolonged doses can generate a significant accumulation of this metal resulting in kidney failure [36]. Regarding the cardiovascular effects, exposure to toxic levels of mercury can cause various illnesses such as an increased heartbeat, irregular pulsations, and even high blood pressure [2]. Some studies have shown a correlation between the intake of mercury through the frequent consumption of fish and the increase in the blood pressure of specific patients. In addition, a specific correlation between mercury exposure and an increased risk of hypertension and myocardial infarction has been detected [37]. The last aspect is the reproductive problems that mercury exposure can cause. Some authors have shown that this metal can negatively affect the reproductive functions of men and women. Other studies suggest that long mercury exposure can lead to a generation of congenital disabilities because of its toxicity [2].
Some investigations have demonstrated mercury’s negative effects based on previous health issues. In the SH, some relevant health issues are related to the main anthropogenic atmospheric mercury sources. In Palu city in Indonesia, a country partially in the SH, research has detected a high GEM level in that city close to ASGM activities, reaching between 2.06 and 375 ng/m3. It was determined that the high exposure to GEM was an elevated human health risk for residents [38]. In Bolivia, because of ASGM processes, there are high risks to the population’s health in the mountain zones close to the mining operations. The two mining sites studied had levels of mercury vapour that exceeded the EPA’s recommended concentration [39]. In addition, settlements close to the mining operations have detected levels of Hg and some families burn Hg amalgam in their homes without respiratory protection. In that research, the authors concluded that there was a high risk of cancerous and non-cancerous health problems for the population in the studied area [39].
A study in Tanzania showed the high risk faced by gold mine workers and local communities because of Hg exposure [40]. Research participants who were highly exposed to Hg reported medical symptoms, such as tremors in diverse body parts, sensory disturbances, and coordination problems. Long-term mercury exposure causes symptoms related to central and peripheral nervous system damage [40]. Another case is Chile, where a study of children in rural schools showed that long-term mercury exposure can impact children’s motor skills [41]. The rural areas studied were near ASGM activities, where the population has been constantly exposed to atmospheric mercury even though mining companies have implemented diverse environmental management strategies to reduce pollution [42]. The authors argued that children exposed to Hg burning could have pathological pure motor skills because of an alteration in the central nervous system [41]. Another case is Ecuador, where a study on children in a mining area detected high mercury concentrations. The mean mercury concentration in the blood was close to 3.23 µg/L [43]. The authors identified these elevated mercury levels in children in urban and rural areas near gold mining activities [44]. In another place in Ecuador, Portovelo, an investigation detected a high mercury concentration level in the air. Researchers identified that in urban areas near the mining operations, atmospheric mercury levels were beyond the defined dangerous concentration level (around 200 ng/m3) [45]. In the places described in this section, the health of the populations are at risk. The levels of mercury detected in areas close to ASGM activities, in most cases, exceeded current regulations. In this sense, educating the population about the negative effects of mercury exposure is essential. Further, there is a need to prevent the adverse effects on communities, especially the neurotoxicity effect it can have on children [44][45].
This entry is adapted from the peer-reviewed paper 10.3390/atmos13081226
References
- Balakrishnan, K.; Dey, S.; Gupta, T.; Dhaliwal, R.S.; Brauer, M.; Cohen, A.J.; Stanaway, J.D.; Beig, G.; Joshi, T.K.; Aggarwal, A.N.; et al. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The Global Burden of Disease Study 2017. Lancet Planet. Health 2019, 3, e26–e39.
- Kim, K.-H.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385.
- Chen, C.Y.; Driscoll, C.T.; Eagles-Smith, C.A.; Eckley, C.S.; Gay, D.A.; Hsu-Kim, H.; Keane, S.E.; Kirk, J.L.; Mason, R.P.; Obrist, D.; et al. A Critical Time for Mercury Science to Inform Global Policy. Environ. Sci. Technol. 2018, 52, 9556–9561.
- Toda, E.; Have, C.T.; Pacyna, J.M. The Minamata Convention. Chem. Int. 2020, 42, 10–18.
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An updated review of atmospheric mercury. Sci. Total Environ. 2019, 707, 135575.
- Diéguez, M.C.; Bencardino, M.; García, P.E.; D’Amore, F.; Castagna, J.; De Simone, F.; Soto Cárdenas, C.; Ribeiro Guevara, S.; Pirrone, N.; Sprovieri, F. A multi-year record of atmospheric mercury species at a background mountain station in Andean Patagonia (Argentina): Temporal trends and meteorological influence. Atmos. Environ. 2019, 214, 116819.
- Slemr, F.; Martin, L.; Labuschagne, C.; Mkololo, T.; Angot, H.; Magand, O.; Magand, O.; Garat, P.; Ramonet, M.; Bieser, J. Atmospheric mercury in the Southern Hemisphere—Part 1: Trend and inter-annual variations in atmospheric mercury at Cape Point, South Africa, in 2007–2017, and on Amsterdam Island in 2012–2017. Atmos. Chem. Phys. 2020, 20, 7683–7692.
- Wright, L.; Zhang, L.; Marsik, F.J. Overview of mercury dry deposition, litterfall, and throughfall studies. Atmos. Chem. Phys. 2016, 16, 13399–13416.
- Gworek, B.; Dmuchowski, W.; Baczewska, A.H.; Brągoszewska, P.; Bemowska-Kałabun, O.; Wrzosek-Jakubowska, J. Air Contamination by Mercury, Emissions and Transformations—A Review. Water Air Soil Pollut. 2017, 228, 123.
- Subir, M.; Ariya, P.A.; Dastoor, A.P. A review of uncertainties in atmospheric modeling of mercury chemistry I. Uncertainties in existing kinetic parameters e Fundamental limitations and the importance of heterogeneous chemistry. Atmos. Environ. 2011, 45, 5664–5676.
- Si, L.; Ariya, P.A. Recent Advances in Atmospheric Chemistry of Mercury. Atmosphere 2018, 9, 76.
- Zhang, Y.; Bocquet, M.; Mallet, V.; Seigneur, C.; Baklanov, A. Real-time air quality forecasting, part I: History, techniques, and current status. Atmos. Environ. 2012, 60, 632–655.
- Zaid Abualkishik, A. A comparative study on the software architecture of WRF and other numerical weather prediction models. J. Theor. Appl. Inf. Technol. 2018, 31, 24.
- Travnikov, O.; Angot, H.; Artaxo, P.; Bencardino, M.; Bieser, J.; D’Amore, F.; Dastoor, A.; De Simone, F.; DIéguez, M.C.; Dommergue, A.; et al. Multi-model study of mercury dispersion in the atmosphere: Atmospheric processes and model evaluation. Atmos. Chem. Phys. 2017, 17, 5271–5295.
- Angot, H.; Hoffman, N.; Giang, A.; Thackray, C.P.; Hendricks, A.N.; Urban, N.R.; Selin, N.E. Global and Local Impacts of Delayed Mercury Mitigation Efforts. Environ. Sci. Technol. 2018, 52, 12968–12977.
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140.
- Zhang, L.; Zhou, P.; Cao, S.; Zhao, Y. Atmospheric mercury deposition over the land surfaces and the associated uncertainties in observations and simulations: A critical review. Atmos. Chem. Phys. 2019, 19, 15587–15608.
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284.
- Falakdin, P.; Terzaghi, E.; Di Guardo, A. Spatially resolved environmental fate models: A review. Chemosphere 2022, 290, 133394.
- Zhang, P.; Zhang, Y. Earth system modeling of mercury using CESM2—Part 1: Atmospheric model CAM6-Chem/Hg v1.0. Geosci. Model Dev. 2022, 15, 3587–3601.
- AMAP; UN Environment. Technical Background Report for the Global Mercury Assessment 2018; Arctic Monitoring and Assessment Programme: Oslo, Norway; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019; pp. viii + 426.
- Kos, G.; Ryzhkov, A.; Dastoor, A.; Narayan, J.; Steffen, A.; Ariya, P.A.; Zhang, L. Evaluation of discrepancy between measured and modelled oxidized mercury species mercury species. Atmos. Chem. Phys 2013, 13, 4839–4863.
- De Simone, F.; D’Amore, F.; Bencardino, M.; Carbone, F.; Hedgecock, I.M.; Sprovieri, F.; Cinnirella, S.; Pirrone, N. The GOS4M Knowledge Hub: A web-based effectiveness evaluation platform in support of the Minamata Convention on Mercury. Environ. Sci. Policy 2021, 124, 235–246.
- Charv At, P.; Klime, L.; Pospíšil, J.; Kleme, J.; Varbanov, S. An overview of mercury emissions in the energy industry-A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087.
- Steenhuisen, F.; Wilson, S.J. Development and application of an updated geospatial distribution model for gridding 2015 global mercury emissions. Atmos. Environ. 2019, 211, 138–150.
- De Simone, F.; Hedgecock, I.M.; Carbone, F.; Cinnirella, S.; Sprovieri, F.; Pirrone, N. Estimating Uncertainty in Global Mercury Emission Source and Deposition Receptor Relationships. Atmosphere 2017, 8, 236.
- AMAP; UN Environment. Technical Background Report for the Global Mercury Assessment; United Nations Environment Programme: Nairobi, Kenya, 2013.
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964.
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105.
- Shi, Y.; Zhao, A.; Matsunaga, T.; Yamaguchi, Y.; Zang, S.; Li, Z.; Yu, T.; Gu, X. High-resolution inventory of mercury emissions from biomass burning in tropical continents during 2001–2017. Sci. Total Environ. 2019, 653, 638–648.
- Friedli, H.R.; Arellano, A.F.; Cinnirella, S.; Pirrone, N. Mercury emissions from global biomass burning: Spatial and temporal distribution. In Mercury Fate and Transport in the Global Atmosphere; Springer: Boston, MA, USA, 2009; pp. 193–220.
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983.
- Zhang, Y.; Song, Z.; Huang, S.; Zhang, P.; Peng, Y.; Wu, P.; Gu, J.; Dutkiewicz, S.; Zhang, H.; Wu, S.; et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 2021, 12, 3035.
- Peplow, D.; Augustine, S. Neurological abnormalities in a mercury exposed population among indigenous Wayana in Southeast Suriname. Environ. Sci. Process. Impacts 2014, 16, 2415–2422.
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737.
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508.
- Guallar, E.; Sanz-Gallardo, M.I.; Veer, P.V.T.; Bode, P.; Aro, A.; Gómez-Aracena, J.; Kark, J.D.; Riemersma, R.A.; Martín-Moreno, J.M.; Kok, F.J. Mercury, Fish Oils, and the Risk of Myocardial Infarction. N. Engl. J. Med. 2009, 347, 1747–1754.
- Nakazawa, K.; Nagafuchi, O.; Kawakami, T.; Inoue, T.; Elvince, R.; Kanefuji, K.; Nur, I.; Napitupulu, M.; Basir-Cyio, M.; Kinoshita, H.; et al. Human health risk assessment of atmospheric mercury inhalation around three artisanal small-scale gold mining areas in Indonesia. Environ. Sci. Atmos. 2021, 1, 423–433.
- Pavilonis, B.; Grassman, J.; Johnson, G.; Diaz, Y.; Caravanos, J. Characterization and risk of exposure to elements from artisanal gold mining operations in the Bolivian Andes. Environ. Res. 2017, 154, 1–9.
- Bose-O’reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health assessment of artisanal gold miners in Tanzania. Sci. Total Environ. 2009, 408, 796–805.
- Ohlander, J.; Huber, S.M.; Schomaker, M.; Heumann, C.; Schierl, R.; Michalke, B.; Jenni, O.G.; Caflisch, J.; Muñoz, D.M.; von Ehrenstein, O.S.; et al. Mercury and neuromotor function among children in a rural town in Chile. Int. J. Occup. Environ. Health 2016, 22, 27–35.
- Leiva González, J.; Onederra, I. Environmental Management Strategies in the Copper Mining Industry in Chile to Address Water and Energy Challenges-Review. Mining 2022, 2, 197–232.
- Hrubá, F.; Strömberg, U.; Černá, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppová, K.; Kos, A.; Krsková, A.; et al. Blood cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34.
- Laborde, A.; Tomasina, F.; Bianchi, F.; Bruné, M.N.; Buka, I.; Comba, P.; Corra, L.; Cori, L.; Duffert, C.M.; Harari, R.; et al. Children’s health in Latin America: The infuence of environmental exposures. Environ. Health Perspect. 2015, 123, 201–209.
- González-Carrasco, V.; Velasquez-Lopez, P.C.; Olivero-Verbel, J.; Pájaro-Castro, N. Air Mercury Contamination in the Gold Mining Town of Portovelo, Ecuador. Bull. Environ. Contam. Toxicol. 2011, 87, 250–253.
This entry is offline, you can click here to edit this entry!