Gastrointestinal tract microbiota plays a key role in the regulation of the pathogenesis of several gastrointestinal diseases. In particular, the viral fraction, composed essentially of bacteriophages, influences homeostasis by exerting selective pressure on the bacterial communities living in the tract. The inflamed gut is associated with an SOS response regulated by an increase in resident intestinal pathogenic bacteria, loss of phage diversity, and induction of prophages.
- bacteriophage
- microbiome
- gastrointestinal tract
- dysbiosis
Most phages in the human intestine are temperate, suggesting that gut microbiome is fairly stable in the gastrointestinal tract. This idea has led to the establishment of a global intestinal phagome, showing a correlation between phages and health status, and his role in maintaining the structure and function of the intestinal microbiome [1]. This stability allows the maintenance of other microorganisms of the intestinal microbiota [2]. Interestingly, the prevalence of healthy intestinal phagome was found to be significantly altered in patients with ulcerative colitis and Crohn's disease. Bacteriophage richness, measured by the number of taxa per sample, was showed to be higher in patients undergoing these diseases, while bacterial richness decreased concomitantly [3].
The inflamed gut is associated with an SOS response regulated by an increase in resident intestinal pathogenic bacteria, loss of phage diversity, and induction of prophages. Several environmental conditions trigger the response to SOS bacterial stress, such as ultraviolet light, drugs or antibiotics [4]. As a clear example, most orally administered antibiotics alter the intestinal microbiota, promoting dysbiosis (Figure 1) [5].
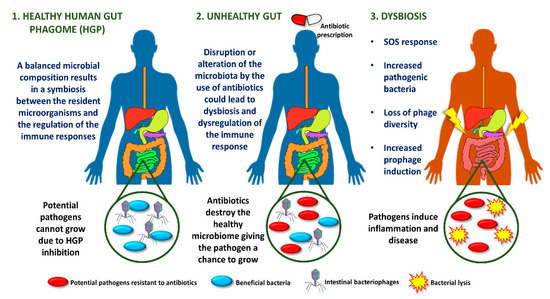
Figure 1. Antibiotic-associated intestinal dysbiosis.
Phages can contribute as vectors for horizontal gene transfer. The high induction of prophages during inflammation favors the horizontal gene transfer mechanism among its bacterial hosts, which increases the rate of genetic recombination and diversification. This process actively shapes the evolution of bacteria modifying virulence and antibiotic resistance factors. In addition, phage genes can indirectly increase bacterial toxin production, having implications in adhesion, colonization, and invasion of the immune response [5].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8091420
References
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405.
- Knowles, B.; Silveira, C.B.; Bailey, B.A.; Barott, K.; Cantu, V.A.; Cobian-Guemes, A.G.; Coutinho, F.H.; Dinsdale, E.A.; Felts, B.; Furby, K.A.; et al. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470.
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460.
- Imamovic, L.; Balleste, E.; Martinez-Castillo, A.; Garcia-Aljaro, C.; Muniesa, M. Heterogeneity in phage induction enables the survival of the lysogenic population. Environ. Microbiol. 2016, 18, 957–969.
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977.
