The ability to measure and monitor the concentration of specific chemical and/or gaseous species (i.e., “analytes”) is the main requirement in many fields, including industrial processes, medical applications, and workplace safety management. As a consequence, several kinds of sensors have been developed in the modern era according to some practical guidelines that regard the characteristics of the active (sensing) materials on which the sensor devices are based. These characteristics include the cost-effectiveness of the materials’ manufacturing, the sensitivity to analytes, the material stability, and the possibility of exploiting them for low-cost and portable devices. Consequently, many gas sensors employ well-defined transduction methods, the most popular being the oxidation (or reduction) of the analyte in an electrochemical reactor, optical techniques, and chemiresistive responses to gas adsorption. In recent years, many of the efforts devoted to improving these methods have been directed towards the use of certain classes of specific materials.
- gas sensors
- ionic liquids
- metal–organic frameworks
- MOF-based composites
- optical sensors
- chemiresistors
- electrochemical sensors
- oxygen
- hydrogen
- chemical sensing
1. Introduction
| Sensor Type | Examples | Principle of Operation |
|---|---|---|
| Electrochemical | Amperometric, ChemFET |
Analyte molecules are involved in the redox reaction at the working electrode of an electrochemical cell, modulating the electrical current. |
| Electrical | Chemoresistors | Adsorbed molecules of the target gas interact with oxygen species adsorbed on the surface of a nanoparticulated semiconductor, modifying its charge depletion regions and its electrical conductivity. |
| Gravimetric | Surface acoustic waves, piezoelectric |
A vibration resonance frequency is modified due to the adsorption of the target analyte. The shift in resonance frequency quantifies the analyte concentration. |
| Thermochemical | Catalytic bead sensors | The target gas is burnt, causing a temperature rise that changes the resistance of the detecting element of the sensor proportional to the concentration of combusted gas. |
| Optical | Absorptive Reflective Fluorescence-based |
Adsorbed molecules of the target gas modify in several ways the optical properties of the sensing material (e.g., reflectivity, optical transmission, fluorescence spectrum and/or lifetime, etc.). |
2. Ionic Liquids in Amperometric Gas Sensing—Recent Developments
| Analyte | Ionic Liquid | Electrode | Analyte Concentration | Ref. | |
|---|---|---|---|---|---|
| O2 | |||||
| [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 1–20% | [83] | ||
| [C2mim][NTf2] and [C4mpyrr][NTf2] |
Screen-printed (SP) electrodes | 10–100% and 0.1–5% | [84] | ||
| [N8,2,2,2][NTf2] | Pt MATFE | 10–100% | [85] | ||
| [C2mim][NTf2] | Pt microdisk and Pt MATFE | 0.1–100% | [86] | ||
| [MOmim][PF6] | Au microchannel electrode |
5000–25,000 ppm | [87] | ||
| [Bmim][BF4] | Au interdigitated electrodes | 20–100% | [88] | ||
| [C4mpyrr][NTf2] | Au on porous PTFE substrate | 5–20% | [89] | ||
| [C2mim][NTf2] and [C4mim][PF6] |
SP electrodes (graphite) | 0.1–20% and 100% | [90] | ||
| [C4mpyrr][NTf2] | Au microchannel electrode | 50–400 ppm and 2000–5000 ppm |
[91] | ||
| [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 5–20% | [92] | ||
| [C4mpyrr][NTf2] | Interdigitated electrodes | 1400–4800 ppm | [93] | ||
| [C4mim][PF6], [C2mim][PF6] and [C5mim][PF6] | Pt interdigitated electrodes | 0–100% | [94] | ||
| [C4mim][BF4] | Planar electrodes | 20–100% | [95] | ||
| [Bmim][BF6] | Pt planar electrodes modified by NiCo2O4/rGO/[Bmim][BF6] composite | 20–100% | [96] | ||
| [C4mpn][Br] | Pt microelectrodes, 1% Ag-coated chitosan added to the IL | 20–100% | [97] | ||
| [Bmim][BF4] | SPE, solid polymer electrolyte (PTFE/Carbon nanotubes/IL) | 2.1–12.6% | [98] | ||
| [Emmim][TFSI] and [Bmim][TFSI] |
Pt electrodes, IL + reduced graphene (rGO) + α-Fe2O3 electrolyte | 20–100% | [99] | ||
| [C2mim][NTf2] | IL membrane on Au-TFE | 20–100% | [100] | ||
| [C2mim][NTf2] added with Poly[DADMA][NTf2] |
IL/poly(IL) membrane on Au-TFE | 20–100% | [100] | ||
| O2 and NH3 | [C2mim][BF4] and [C4mim][BF4] |
Gel polymer electrolyte (ILs in PVDF) between planar electrodes | 1–20% for O2; 1–10 ppm for NH3 | [101] | |
| O2 and H2 | [Bmpy][NTf2] | Planar Pt-Ni alloy electrodes | 500–5000 ppm for O2; 500–6250 ppm for H2 | [102] | |
| H2 | |||||
| [C4mim][NTf2] and [C4mpyrr][NTf2] |
Clark-type sensor with polycrystalline Pt gauze |
0.05–1.25% | [103] | ||
| [C4mim]Cl | Pd deposited on carbon gas diffusion electrode | 1–5% | [104] | ||
| [Bmpy][NTf2] | [Bmpy][NTf2] on Pt/C/Nafion screen-printed electrode | 2000–10,000 ppm | [105] | ||
| [C2mim][NTf2] | Au microchannel electrodes with electrodeposited Pt nanoparticles | 0.1–10% | [106] | ||
| NH3 | |||||
| [C2mim][NTf2] | Pt SPE, TFE, MATFE, and microdisk | 10–100 ppm | [107] | ||
| [C2mim][NTf2] | SP electrode, thin-film electrode (TFE), microarray thin-film electrode (MATFE), and microdisk. | 10–100 ppm | [108] | ||
| [C2mim][NTf2] | Pt MATFE | 10–100 ppm | [109] | ||
| [C2mim][NTf2] | Pt-based MATFE (with different morphologies) | 1–2 ppm LODs (depending on the morphology) | [109] | ||
| NH3 and HCl | [C2mim][NTf2] and [C4mpyrr][NTf2] | Au microchannel electrodes | 20–100 ppm | [110] | |
| VOC (in air) | [C4mpyrr][NTf2] | Clark-type sensor with polycrystalline Pt gauze | 200–3000 ppm of acetaldehyde | [111] | |
| CO2 | [Bmpy][NTf2] | Au microchannel electrodes with electrodeposited Cu nanoparticles | 0.14–11% | [112] | |
| Hexanaldehyde (HA) | [Bmim][OH] | Pt microelectrodes | 2–300 ppm (HA in squalene) | [113] | |
| C6H6 and HCHO | [C2mim][EtSO4] | IL and ionogel (IL in poly(N-isopropylacrylamide)) between interdigitated electrodes | 10–50 ppm | [114] | |
| SO2 | [C4mpyrr][NTf2] | TFEs and MATFEs | 1–10 ppm | [115] | |
| H2O (humidity) | [Bmim][DCA] | IL incorporated in gels on interdigitated electrodes | 30–70% RH | [116] | |
| Ethanol | [Bmim][HSO4] | IL on Au screen-printed electrode | 1–10% | [117] | |
| NO2 | [Bmim][NTf2] | Solid polymer electrolyte (PVDF + IL) on screen-printed electrodes | 1–10 ppm | [118] | |
| [Bmim][BF4] | Solid polymer electrolyte (ionic liquid (IL), carbon nanotubes + polyaniline + IL) on SP electrodes | 0–700 ppm | [119] | ||
| Ethylene (C2H4) | [Bmim][NTf2] | Solid polymer electrolyte (PVDF + IL) on SP electrodes | 100–500 ppm | [120] | |
3. Metal–Organic-Framework-Based Composites in Gas Sensing—Recent Developments
3.1. General Properties of Metal–Organic Frameworks
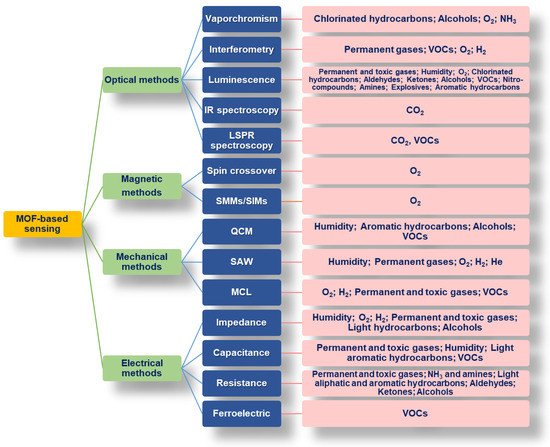
3.2. MOF-Based Sensors Using Gravimetric and Mechanical Methods
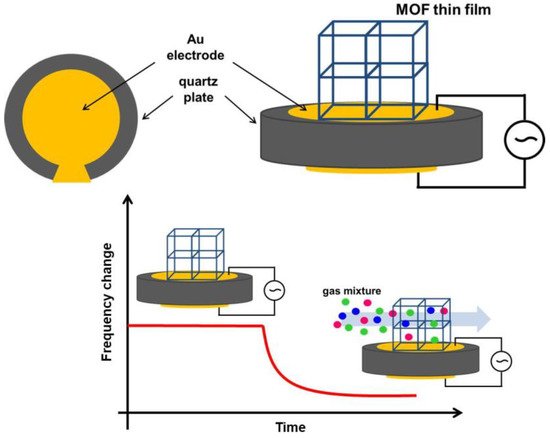
3.2.1. Quartz Crystal Microbalance (QCM)-Based Sensors
3.3.2. Surface Acoustic Wave Sensors (SAWS)
3.2.3. Microcantilever-Based Sensors (MCLs)
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors10080290
