Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Liquid crystals (LCs) are phase transition materials that exist between the liquid and the crystal states, and they can flow as liquids and also have properties such as birefringence of crystals. LCs have been widely used as sensitive elements to construct LC biosensors based on the principle that specific bonding events between biomolecules can affect the orientation of LC molecules.
- liquid crystals
- LC-based biosensors
- microfluidics
1. Introduction
Liquid crystals (LCs) are phase transition materials that exist between the liquid and the crystal states, and they can flow as liquids and also have properties such as birefringence of crystals [1]. LCs can respond readily to external stimuli such as electromagnetic fields [2][3][4][5][6], pressure [7][8][9], surface effects [10][11][12][13], optical properties [14][15], temperature [16][17][18], and chemical analytes [19][20]. Changes in these factors can be monitored by various characterization techniques. Since LCs have superiorities in sensitivity, reactivity, and fabrications, research works on LCs have been expanded widely to address current technological and scientific challenges [21][22]. Accordingly, LC-based sensing techniques have gradually attracted more and more research attention.
LC molecules can be applied to serve as sensitive materials to respond to environmental changes or the occurrence of binding events. LCs generate macroscopic signals by magnifying and transforming molecular events in their surroundings [23]. The response properties of LCs have been extensively investigated in the context of a variety of sensing applications based on the molecular orientation. These properties form the foundation of LC-based sensing platforms [24][25][26][27][28][29]. A popular biosensing approach employs the optical birefringence properties of LC materials. Signal sensors can detect changes in the phases and the polarization states, since the refractive indices (ordinary and extraordinary refractive indices) vary with the direction of the light beam propagating along LCs. Interactions between biological receptors and ligands change the orientation of LC molecules, and this mechanism allows LCs to respond to biological or chemical materials [30][31][32][33][34]. In addition, LC molecules on the surface can transmit variations in their orientations within a vicinity (maximum 100 μm) to amplify the sensing signal [35]. Considering the optical birefringence properties and the high sensitivity to surface–interface interactions, LC biosensors can avoid the drawbacks of traditional biosensors, such as complex operations and the need for labeling biotargets [36]. Consequently, signals generated by LCs can be used to report subtle molecular events and external stimuli, and a number of materials-based optoelectronic signal transduction systems have been developed for LC-based biosensors [37][38][39].
In response to the rapid advancements of the microfluidic technology, optofluidic systems that combine optics and microfluidic devices have steadily demonstrated their distinct merits and potentials. The microfluidic technique is very important in the biomedical detection since it enables all the functions, including the preparation of biological samples [40], the measurement of concentrations [41][42], the monitoring of biochemical reactions [43][44][45][46], and the detection of products [47], to be implemented on a single biochip. Due to the vital role of the optical technologies in the biomedical detection, the integration of sample analysis, detection, and data transfer significantly enhances the efficiency. Moreover, fluids are excellent carriers of the light and matters, meaning that liquids with varying optical properties can be employed to manipulate the light and convey matters [48]. LCs have both optical anisotropy and liquid shapes, and this makes them perfectly suited for detecting and analyzing extremely small samples in biomedicine and chemistry.
2. Principle of LC-Based Biosensors
The mesophase of LCs is defined by the difference in conditions that create it. LCs can be categorized into two groups: lyotropic liquid crystals (LLCs) and thermotropic liquid crystals (TLCs). LLCs are composed of solvents and amphiphilic compounds. The phase transition of the LLCs depends on both the concentration and the temperature. The amphiphilic feature of one end being hydrophilic and the other end being hydrophobic is required for the lyotropic LCs, and the fabrication is dependent on the interactions between the amphiphilic molecules [49]. In contrast, the TLCs can only develop a liquid crystal phase within a limited temperature range (Figure 1a). Such LCs take on a more turbid appearance at the melting point. They continue to heat up until the clearing point is reached, at which point the LCs become transparent. According to the arrangement of the LC molecules, thermotropic LCs can be divided into three types: nematic liquid crystals (NLCs), cholesteric liquid crystals (CLCs), and smectic liquid crystals (SLCs) (Figure 1b). NLC molecules possess low van der Waals forces, excellent fluidity, and optical birefringence. Due to the high sensitivity to the environmental changes, NLC materials are widely investigated [50]. CLCs, also known as chiral NLC phases, are the chiral variants of orientationally ordered NLC phases. CLC molecules have a director that is periodically rotated along the perpendicular axis; the orientation rotates periodically about a vertical axis, generating a helical superstructure. The CLC possesses exceptional optical properties, including optical rotation, circularly polarized light dichroism, and selective light scattering [51]. An SLC molecule is rod- or strip-shaped. Molecules in SLCs tend to align in layers, and the layers stack on each other. It is noted that all LCs discussed in this research are thermotropic.
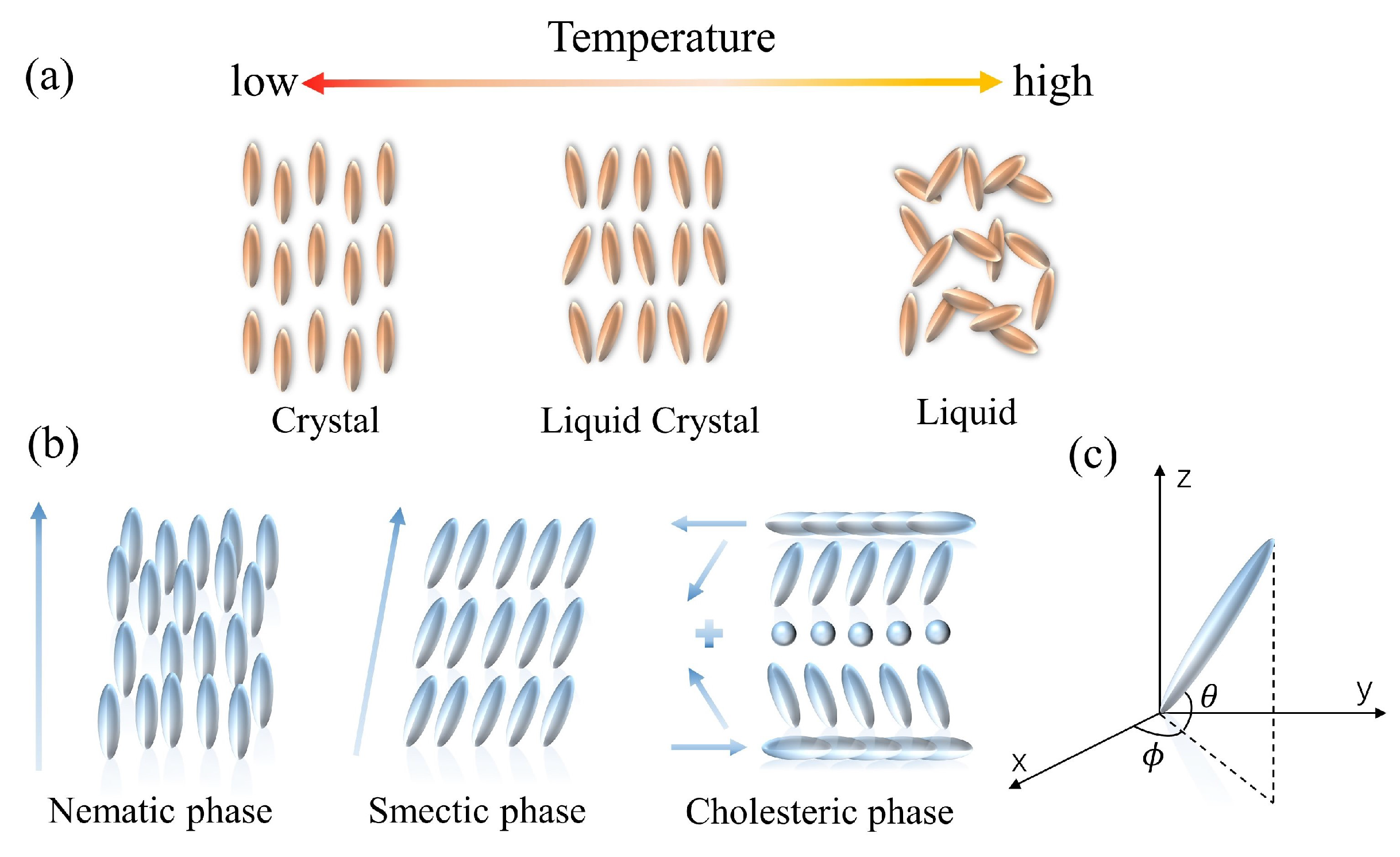
Figure 1. Schematic of the properties of LC molecules. (a) The arrangement of thermotropic LC molecules. (b) The variation of thermotropic LC molecules with temperature. (c) Illustration of LC directors in the Cartesian coordinate.
2.1. Optical Anisotropy
LC molecules exhibit the anisotropy in terms of refractive index, dielectric properties, elastic coefficient, etc., due to the structural peculiarities of LCs (between crystals and liquids) [52][53][54][55]. The anisotropy in the refractive index is the most commonly used optical birefringence characteristic. Plane polarized light is decomposed into two linearly polarized rays with different propagation rates, which are called ordinary (no) and extraordinary (ne) rays, respectively [56]. The birefringence property of LC can be described as the difference between the refractive indices of no and ne. This phenomenon will cause a phase change as the light beam outputs from the LC medium. The phase difference is known as the optical retardation δ, and is given by [57]:
where λ is the wavelength of the light and d is the thickness of the medium. Therefore, LC systems are able to regulate the polarization of light due to the birefringence and convert this into an optical image visible to the naked eye, which can be viewed using a polarized optical microscope (POM).
The birefringence on the surface of LC molecules can cause changes in the optical image, and this means that the monitoring of changes in the optical signal image using a POM allows the indirect detection of the variations in the alignment of LC molecules. Due to the interference of two orthogonal rays, the optical texture of LCs is visible through crossed polarizers. The linearly polarized light beam cannot get through in the case of the homeotropic alignment of the LC molecules, and this will result in dark optical images. The director configuration of LCs restricted inside particular geometries can be identified using the POM, by observing patterns produced by the interaction between the polarized light and LCs [58]. The transmitted light has the following intensity when LCs are sandwiched between crossed polarizers:
where I0 is the initial intensity of the light and φ is the angle of the LC orientations relative to the polarizer. This formula provides a good explanation of the fact that LC molecules can produce varied optical images when observed under the polarized light [59]. When LCs are positioned between two crossed polarizers, the intensity of the transmitted light I = 0 for the homeotropic alignment of LCs (δ = 0), and a dark optical image is observed. When the LC orientations are disturbed, δ≠0, I≠0, a bright image will be observed.
2.2. Orientations of LCs
Owing to the interaction force between LC molecules and hydrogen bonding, the long axes of LCs tend to align parallel to each other. In addition, the orientation of molecules follows a certain order, and the preferred direction is called the director of phase, which is the average direction that molecules point [60]. The unit vector n→ is used to describe the direction of the preferred orientation, and the spatial orientation of the director is normally specified by the polar angle θ and the azimuth angle φ, as shown in Figure 1c. In general, it can be used to characterize the macroscopic structure and the condition of LCs, as it describes the arrangement direction of LC molecules in space. While LCs are in the dynamic motion, they are oriented along the director for a longer period. Accordingly, an average indicator is required to quantify the degree of LC ordering, i.e., the order parameter S [61], which can be defined as follows:
where θ is the angle between the long axis of LC molecules and the director. The long axis orientation of molecules in isotropic liquids is disordered, and thus S = 0. At the state of fully parallel LC molecules, we have S = 1. Consequently, the order parameter S, which is utilized to quantify the degree of orientation of molecules with respect to the director, is an important physical property for LCs. However, S is temperature-dependent and will decrease with the increase of the temperature, because LCs will vary from an anisotropic crystalline state to an isotropic liquid state accordingly [11]. The LC director is essential in producing LC biosensors since the spatial orientation significantly affects the characteristics of LCs. As a result of the contact force between LC molecules, an ordered arrangement can be built to realize the function of sensing, and the orientations of LCs will be readjusted by external variables.
This entry is adapted from the peer-reviewed paper 10.3390/bios12080639
References
- Kalita, P.; Shukla, S.S.; Singh, R.K.; Bhattacharjee, A. Potential liquid crystal-based biosensor depending on the interaction between liquid crystals and proteins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119634.
- Oh-e, M.; Zheng, D.Y. Newly discovered dimensional effects of electrodes on liquid crystal THz phase shifters enable novel switching between in-plane and out-of-plane. Sci. Rep. 2022, 12, 5482.
- Prévôt, M.E.; Nemati, A.; Cull, T.R.; Hegmann, E.; Hegmann, T. A zero-power optical, ppt-to ppm-level toxic gas and vapor sensor with image, text, and analytical capabilities. Adv. Mater. Technol. 2020, 5, 2000058.
- Tefelska, M.M.; Woliński, T.R.; Ertman, S.; Mileńko, K.; ączkowski, R.; Siarkowska, A.; Domański, A.W. Electric field sensing with photonic liquid crystal fibers based on micro-electrodes systems. J. Light. Technol. 2015, 33, 2405–2411.
- Zheng, Z.G.; Yuan, C.L.; Hu, W.; Bisoyi, H.K.; Tang, M.J.; Liu, Z.; Sun, P.Z.; Yang, W.Q.; Wang, X.Q.; Shen, D.; et al. Light-patterned crystallographic direction of a self-organized 3d soft photonic crystal. Adv. Mater. 2017, 29, 1703165.
- Mathews, S.; Farrell, G.; Semenova, Y. Directional electric field sensitivity of a liquid crystal infiltrated photonic crystal fiber. IEEE Photonics Technol. Lett. 2011, 23, 408–410.
- Baratta, M.; De Filpo, G.; Tursi, A.; Mashin, A.I.; Nicoletta, F.P. Polymer Dispersed Liquid Crystals with elongated droplets as novel pressure sensors. Liq. Cryst. 2021, 49, 657–665.
- Kumari, S.; Singh, S. Influence of pressure on the B2I phase transition of a banana-shaped liquid crystal. Liq. Cryst. 2014, 41, 522–529.
- Prasad, S.K.; Gupta, V.K.; Rao, D.S.; Lobo, C.V. Effect of pressure on the dynamics of the photostimulated orientational ordering transition in a liquid crystal. Phys. Rev. E 2005, 72, 021705.
- Brake, J.M.; Daschner, M.K.; Luk, Y.Y.; Abbott, N.L. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science 2003, 302, 2094–2097.
- Blinov, L.M. Structure and Properties of Liquid Crystals; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; Volume 123.
- Tan, H.; Li, X.; Liao, S.; Yu, R.; Wu, Z. Highly-sensitive liquid crystal biosensor based on DNA dendrimers-mediated optical reorientation. Biosens. Bioelectron. 2014, 62, 84–89.
- Zhou, C.H.; Zi, Q.J.; Wang, J.; Zhao, W.Y.; Cao, Q. Determination of alkaline phosphatase activity and of carcinoembryonic antigen by using a multicolor liquid crystal biosensor based on the controlled growth of silver nanoparticles. Microchim. Acta 2019, 186, 25.
- Zhao, D.; Peng, Y.; Xu, L.; Zhou, W.; Wang, Q.; Guo, L. Liquid-crystal biosensor based on nickel-nanosphere-induced homeotropic alignment for the amplified detection of thrombin. ACS Appl. Mater. Interfaces 2015, 7, 23418–23422.
- Vicari, L. Optical Applications of Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2016.
- Wang, F.; Liu, Y.; Lu, Y.; Zhang, L.; Ma, J.; Wang, L.; Sun, W. High-sensitivity Fabry–Perot interferometer temperature sensor probe based on liquid crystal and the Vernier effect. Opt. Lett. 2018, 43, 5355–5358.
- Sun, B.; Huang, Y.; Luo, D.; Wang, C.; He, J.; Liao, C.; Yin, G.; Zhou, J.; Liu, S.; Zhao, J.; et al. Broadband thermo-optic switching effect based on liquid crystal infiltrated photonic crystal fibers. IEEE Photonics J. 2015, 7, 1–7.
- Hu, D.J.J.; Lim, J.L.; Cui, Y.; Milenko, K.; Wang, Y.; Shum, P.P.; Wolinski, T. Fabrication and characterization of a highly temperature sensitive device based on nematic liquid crystal-filled photonic crystal fiber. IEEE Photonics J. 2012, 4, 1248–1255.
- Zhang, J.; Su, X.; Yang, D.; Luan, C. Label-free liquid crystal biosensor for cecropin B detection. Talanta 2018, 186, 60–64.
- Bera, T.; Fang, J. Polyelectrolyte-coated liquid crystal droplets for detecting charged macromolecules. J. Mater. Chem. 2012, 22, 6807–6812.
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078.
- Hussain, Z.; Qazi, F.; Ahmed, M.I.; Usman, A.; Riaz, A.; Abbasi, A.D. Liquid crystals based sensing platform-technological aspects. Biosens. Bioelectron. 2016, 85, 110–127.
- Kim, H.R.; Kim, J.H.; Kim, T.S.; Oh, S.W.; Choi, E.Y. Optical detection of deoxyribonucleic acid hybridization using an anchoring transition of liquid crystal alignment. Appl. Phys. Lett. 2005, 87, 143901.
- Liao, S.; Ding, H.; Wu, Y.; Wu, Z.; Shen, G.; Yu, R. Label-free liquid crystal biosensor for L-histidine: A DNAzyme-based platform for small molecule assay. Biosens. Bioelectron. 2016, 79, 650–655.
- Amin, N.u.; Siddiqi, H.M.; Kun Lin, Y.; Hussain, Z.; Majeed, N. Bovine serum albumin protein-based liquid crystal biosensors for optical detection of toxic heavy metals in water. Sensors 2020, 20, 298.
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938.
- Chen, C.H.; Yang, K. A liquid crystal biosensor for detecting organophosphates through the localized pH changes induced by their hydrolytic products. Sens. Actuators B Chem. 2013, 181, 368–374.
- Ireland, P.; Jones, T. Liquid crystal measurements of heat transfer and surface shear stress. Meas. Sci. Technol. 2000, 11, 969.
- Nguyen, D.K.; Jang, C.H. An acetylcholinesterase-based biosensor for the detection of pesticides using liquid crystals confined in microcapillaries. Colloids Surf. B Biointerfaces 2021, 200, 111587.
- Ping, J.; Qi, L.; Wang, Q.; Liu, S.; Jiang, Y.; Yu, L.; Lin, J.M.; Hu, Q. An integrated liquid crystal sensing device assisted by the surfactant-embedded smart hydrogel. Biosens. Bioelectron. 2021, 187, 113313.
- Zhou, L.; Kang, Q.; Fang, M.; Yu, L. Label-free, rapid, and sensitive detection of carboxylesterase using surfactant-doped liquid crystal sensor. J. Mol. Liq. 2019, 296, 111921.
- Ho, W.F.; Chan, H.P.; Yang, K.L. Planar optical waveguide platform for gas sensing using liquid crystal. IEEE Sens. J. 2013, 13, 2521–2522.
- Esteves, C.; Ramou, E.; Porteira, A.R.P.; Moura Barbosa, A.J.; Roque, A.C.A. Seeing the unseen: The role of liquid crystals in gas-sensing technologies. Adv. Opt. Mater. 2020, 8, 1902117.
- Nayani, K.; Rai, P.; Bao, N.; Yu, H.; Mavrikakis, M.; Twieg, R.J.; Abbott, N.L. Liquid crystals with interfacial ordering that enhances responsiveness to chemical targets. Adv. Mater. 2018, 30, 1706707.
- Xue, C.Y.; Yang, K.L. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567.
- Tabatabaei, M.S.; Islam, R.; Ahmed, M. Applications of gold nanoparticles in ELISA, PCR, and immuno-PCR assays: A review. Anal. Chim. Acta 2021, 1143, 250–266.
- Duan, R.; Li, Y.; Li, H.; Yang, J. Detection of heavy metal ions using whispering gallery mode lasing in functionalized liquid crystal microdroplets. Biomed. Opt. Express 2019, 10, 6073–6083.
- Geng, J.L.; Song, L. Accurate modelling of nematic liquid crystal under the combination of microwave signal and applied voltage. Liq. Cryst. 2020, 47, 67–75.
- Peccianti, M.; Assanto, G. Signal readdressing by steering of spatial solitons in bulk nematic liquid crystals. Opt. Lett. 2001, 26, 1690–1692.
- Courtney, M.; Chen, X.; Chan, S.; Mohamed, T.; Rao, P.P.; Ren, C.L. Droplet microfluidic system with on-demand trapping and releasing of droplet for drug screening applications. Anal. Chem. 2017, 89, 910–915.
- Warkiani, M.E.; Tay, A.K.P.; Khoo, B.L.; Xiaofeng, X.; Han, J.; Lim, C.T. Malaria detection using inertial microfluidics. Lab Chip 2015, 15, 1101–1109.
- Crevillén, A.G.; Ávila, M.; Pumera, M.; González, M.C.; Escarpa, A. Food analysis on microfluidic devices using ultrasensitive carbon nanotubes detectors. Anal. Chem. 2007, 79, 7408–7415.
- Xu, Y.; Rather, A.M.; Yao, Y.; Fang, J.C.; Mamtani, R.S.; Bennett, R.K.; Atta, R.G.; Adera, S.; Tkalec, U.; Wang, X. Liquid crystal–based open surface microfluidics manipulate liquid mobility and chemical composition on demand. Sci. Adv. 2021, 7, eabi7607.
- Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Verdian, A. Liquid crystal-based biosensors as lab-on-chip tools: Promising for future on-site detection test kits. TrAC Trends Anal. Chem. 2021, 142, 116325.
- Kaziz, S.; Saad, Y.; Gazzah, M.H.; Belmabrouk, H. 3D simulation of microfluidic biosensor for SARS-CoV-2 S protein binding kinetics using new reaction surface design. Eur. Phys. J. Plus 2022, 137, 241.
- Hosic, S.; Murthy, S.K.; Koppes, A.N. Microfluidic sample preparation for single cell analysis. Anal. Chem. 2016, 88, 354–380.
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583.
- Sengupta, A. Topological Microfluidics: Nematic Liquid Crystals and Nematic Colloids in Microfluidic Environment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Demus, D. 100 Years Liquid Crystal Chemistry. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1988, 165, 45–84.
- Hyeon, S.G.; Lee, J.H.; Kim, D.H.; Jeong, H.C.; Oh, B.Y.; Han, J.M.; Lee, J.W.; Seo, D.S. Free residual DC voltage for nematic liquid crystals on solution-derived lanthanum tin oxide film. Liq. Cryst. 2017, 44, 1421–1428.
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-driven control of the helical axis of self-organized soft helical superstructures. Adv. Mater. 2018, 30, 1706512.
- Jannat, M.; Yang, K.L. Liquid crystal-enabled protease inhibition assays developed in a millifluidic device. Sens. Actuators B Chem. 2019, 296, 126595.
- Shah, R.R.; Abbott, N.L. Principles for measurement of chemical exposure based on recognition-driven anchoring transitions in liquid crystals. Science 2001, 293, 1296–1299.
- Lu, M. Liquid crystal orientation induced by Van der Waals interaction. Jpn. J. Appl. Phys. 2004, 43, 8156.
- Kalashnikov, S.; Romanov, N.; Nomoev, A. Study of the properties of liquid crystals modified by nanoparticles. J. Appl. Phys. 2016, 119, 094304.
- Oladepo, S.A. Development and Application of Liquid Crystals as Stimuli-Responsive Sensors. Molecules 2022, 27, 1453.
- Kanwar, A. Measurement of order parameter, birefringence and polarizibility of liquid crystals. J. Opt. 2013, 42, 311–315.
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals; Oxford University Press: Oxford, UK, 1993; Number 83.
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702.
- Qu, R.; Li, G. Overview of Liquid Crystal Biosensors: From Basic Theory to Advanced Applications. Biosensors 2022, 12, 205.
- Goodby, J.W. The nanoscale engineering of nematic liquid crystals for displays. Liq. Cryst. 2011, 38, 1363–1387.
This entry is offline, you can click here to edit this entry!
