Cardiotoxicity is a significant complication of chemotherapeutic agents in cancer patients. Cardiovascular incidents including LV dysfunction, heart failure (HF), severe arrhythmias, arterial hypertension, and death are associated with high morbidity and mortality. Risk stratification of cancer patients prior to initiation of chemotherapy is crucial, especially in high-risk patients for cardiotoxicity. The early identification and management of potential risk factors for cardiovascular side effects seems to contribute to the prevention or minimization of cardiotoxicity. Screening of cancer patients includes biomarkers such as cTnI and natriuretic peptide and imaging measurements such as LV function, global longitudinal strain, and cardiac MRI. Cardioprotective strategies for either primary or secondary prevention include medical therapy such as ACE inhibitors, ARBs, b-blockers, aldosterone antagonists, statins and dexrazoxane, physical therapy, and reduction of chemotherapeutic dosages. However, data regarding dosages, duration of medical therapy, and potential interactions with chemotherapeutic agents are still limited. Collaboration among oncologists, cardiologists, and cardio-oncologists could establish management cardioprotective strategies and approved follow-up protocols in patients with cancer receiving chemotherapy.
- cardiotoxicity
- cancer patients
- chemotherapy
- cardioprotective strategies
- risk factors
- medical therapy
1. Introduction
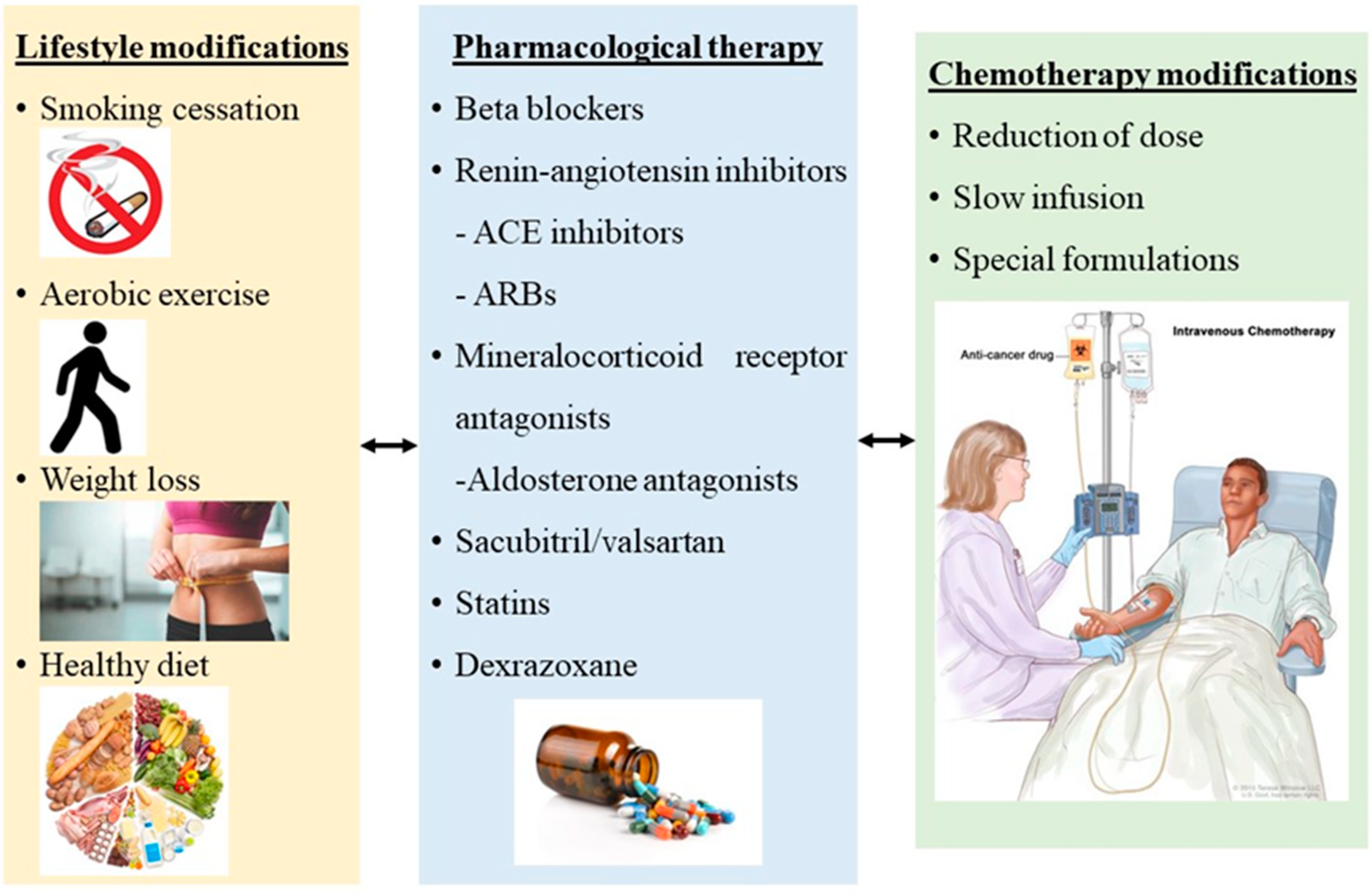
2. Preventive Strategies
3. Primary Prevention of Cardiotoxicity
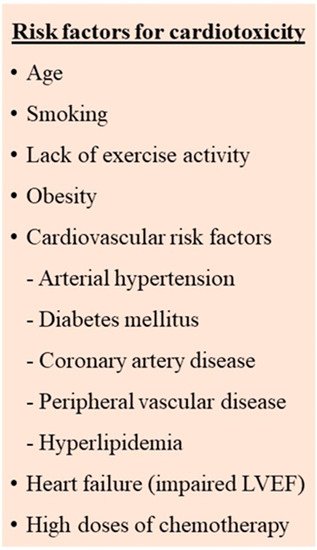
3.1. Identification and Management of Cardiotoxicity Risk Factors
3.2. Cardiovascular Assessment
3.3. Cardioprotective Medical Therapy
This entry is adapted from the peer-reviewed paper 10.3390/jcdd9080259
References
- Page, E.; Assouline, D.; Brun, O.; Coeffic, D.; Fric, D.; Winckel, P. Cardiac dysfunction in clinical trials of trastuzumab. J. Clin. Oncol. 2002, 20, 4119.
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801.
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403.
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661.
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190.
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646.
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130.
- Curigliano, G.; Mayer, E.L.; Burstein, H.J.; Winer, E.P.; Goldhirsch, A. Cardiac toxicity from systemic cancer therapy: A comprehensive review. Prog. Cardiovasc. Dis. 2010, 53, 94–104.
- Valachis, A.; Nilsson, C. Cardiac risk in the treatment of breast cancer: Assessment and management. Breast Cancer 2015, 7, 21–35.
- Blanco, J.G.; Sun, C.L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421.
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939.
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880.
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498.
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816.
- Graffagnino, J.; Kondapalli, L.; Arora, G.; Hawi, R.; Lenneman, C.G. Strategies to Prevent Cardiotoxicity. Curr. Treat. Options Oncol. 2020, 21, 32.
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; das Dores Cruz, F.; Goncalves Brandao, S.M.; Rigaud, V.O.C.; Bocchi, E.A. Carvedilol for prevention of chemotherapy-related cardiotoxicity: The CECCY trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290.
- Tashakori Beheshti, A.; Mostafavi Toroghi, H.; Hosseini, G.; Zarifian, A.; Homaei Shandiz, F.; Fazlinezhad, A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: A double-blind randomized trial. Cardiology 2016, 134, 47–53.
- Elitok, A.; Oz, F.; Cizgici, A.Y.; Kilic, L.; Ciftci, R.; Sen, F.; Bugra, Z.; Mercanoglu, F.; Oncul, A.; Oflaz, H. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol. J. 2014, 21, 509–515.
- Jhorawat, R.; Kumari, S.; Varma, S.C.; Rohit, M.K.; Narula, N.; Suri, V.; Malhotra, P.; Jain, S. Preventive role of carvedilol in Adriamycin-induced cardiomyopathy. Indian J. Med. Res. 2016, 144, 725–729.
- Kheiri, B.; Abdalla, A.; Osman, M.; Haykal, T.; Chahine, A.; Ahmed, S.; Osman, K.; Hassan, M.; Bachuwa, G.; Bhatt, D.L. Meta-Analysis of Carvedilol for the Prevention of Anthracycline-Induced Cardiotoxicity. Am. J. Cardiol. 2018, 122, 1959–1964.
- Kaya, M.G.; Ozkan, M.; Gunebakmaz, O.; Akkaya, H.; Kaya, E.G.; Akpek, M.; Kalay, N.; Dikilitas, M.; Yarlioglues, M.; Karaca, H.; et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. Int. J. Cardiol. 2013, 167, 2306–2310.
- Nabati, M.; Janbabai, G.; Baghyari, S.; Esmaili, K.; Yazdani, J. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J. Cardiovasc. Pharmacol. 2017, 69, 279–285.
- Georgakopoulos, P.; Roussou, P.; Matsakas, E.; Karavidas, A.; Anagnostopoulos, N.; Marinakis, T.; Galanopoulos, A.; Georgiakodis, F.; Zimeras, S.; Kyriakidis, M.; et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: A prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am. J. Hematol. 2010, 85, 894–896.
- Kalay, N.; Basar, E.; Ozdogru, I.; Er, O.; Cetinkaya, Y.; Dogan, A.; Inanc, T.; Oguzhan, A.; Eryol, N.K.; Topsakal, R.; et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 2258–2262.
- Dulin, B.; Abraham, W.T. Pharmacology of carvedilol. Am. J. Cardiol. 2004, 93, 3B–6B.
- Abreu, R.M.; Santos, D.J.; Moreno, A.J. Effects of carvedilol and its analog BM-910228 on mitochondrial function and oxidative stress. J. Pharmacol. Exp. Ther. 2000, 295, 1022–1030.
- Fratta Pasini, A.; Garbin, U.; Nava, M.C.; Stranieri, C.; Davoli, A.; Sawamura, T.; Lo Cascio, V.; Cominacini, L. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J. Hypertens. 2005, 23, 589–596.
- Neaton, J.D.; Grimm, R.H., Jr.; Prineas, R.J.; Stamler, J.; Grandits, G.A.; Elmer, P.J.; Cutler, J.A.; Flack, J.M.; Schoenberger, J.A.; McDonald, R.; et al. Treatment of mild hypertension study. Final results. Treatment of mild hypertension study research group. JAMA 1993, 270, 713–724.
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006, 114, 2474–2481.
- Vaynblat, M.; Shah, H.R.; Bhaskaran, D.; Ramdev, G.; Davis, W.J., 3rd; Cunningham, J.N., Jr.; Chiavarelli, M. Simultaneous angiotensin converting enzyme inhibition moderates ventricular dysfunction caused by doxorubicin. Eur. J. Heart Fail. 2002, 4, 583–586.
- Cardinale, D.; Ciceri, F.; Latini, R.; Franzosi, M.G.; Sandri, M.T.; Civelli, M.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Cavina, R.; et al. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The international CardioOncology society-one trial. Eur. J. Cancer 2018, 94, 126–137.
- Janbabai, G.; Nabati, M.; Faghihinia, M.; Azizi, S.; Borhani, S.; Yazdani, J. Effect of enalapril on preventing anthracycline-induced cardiomyopathy. Cardiovasc. Toxicol. 2017, 17, 130–139.
- Nakamae, H.; Tsumura, K.; Terada, Y.; Nakane, T.; Nakamae, M.; Ohta, K.; Yamane, T.; Hino, M. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005, 104, 2492–2498.
- Bosch, X.; Rovira, M.; Sitges, M.; Domenech, A.; Ortiz-Perez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzó, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362.
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680.
- Pituskin, E.; Mackey, J.R.; Koshman, S.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; Thompson, R.B.; Vos, L.J.; et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J. Clin. Oncol. 2017, 35, 870–877.
- Kanorskiy, S.G.; Pavlovets, V.P. First experience of using valsartan/sacubitril in women with heart failure and breast cancer receiving anthracycline-based adjuvant chemotherapy. Med. Council 2019, 16, 42–48.
- Lipshultz, S.E.; Herman, E.H. Anthracycline cardiotoxicity: The importance of horizontally integrating pre-clinical and clinical research. Cardiovasc. Res. 2018, 114, 205–209.
- Akpek, M.; Ozdogru, I.; Sahin, O.; Inanc, M.; Dogan, A.; Yazici, C.; Berk, V.; Karaca, H.; Kalay, N.; Oguzhan, A.; et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015, 17, 81–89.
- Gianni, L.; Herman, E.H.; Lipshultz, S.E.; Minotti, G.; Sarvazyan, N.; Sawyer, D.B. Anthracycline cardiotoxicity: From bench to bedside. J. Clin. Oncol. 2008, 26, 3777–3784.
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology 2019, 5, 18.
- Davignon, J. Pleiotropic effects of pitavastatin. Br. J. Clin. Pharmacol. 2012, 73, 518–535.
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390.
- Chotenimitkhun, R.; D’Agostino, R., Jr.; Lawrence, J.A.; Hamilton, C.A.; Jordan, J.H.; Vasu, S.; Lash, T.L.; Yeboah, J.; Herrington, D.M.; Hundley, W.G. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can. J. Cardiol. 2015, 31, 302–307.
- Acar, Z.; Kale, A.; Turgut, M.; Demircan, S.; Durna, K.; Demir, S.; Meriç, M.; Ağaç, M.T. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 988–989.
- Abdel-Qadir, H.; Bobrowski, D.; Zhou, L.; Austin, P.C.; Calvillo-Argüelles, O.; Amir, E.; Lee, D.S.; Thavendiranathan, P. Statin exposure and risk of heart failure after anthracycline or trastuzumab-based chemotherapy for early breast cancer: A propensity score-matched cohort study. J. Am. Heart Assoc. 2021, 10, e018393.
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can. J. Cardiol. 2019, 35, 153–159.
- US Food and Drug Administration. Orphan Drug Designations and Approvals. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=441314 (accessed on 1 July 2022).
- Reichardt, P.; Tabone, M.D.; Mora, J.; Morland, B.; Jones, R.L. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: Reevaluating the European labeling. Future Oncol. 2018, 14, 2663–2676.
- Hasinoff, B.B.; Herman, E.H. Dexrazoxane: How it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc. Toxicol. 2007, 7, 140–144.
- Hutchins, K.K.; Siddeek, H.; Franco, V.I.; Lipshultz, S.E. Prevention of cardiotoxicity among survivors of childhood cancer. Br. J. Clin. Pharmacol. 2017, 83, 455–465.
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945.
- Lipshultz, S.E.; Scully, R.E.; Lipsitz, S.R.; Sallan, S.E.; Silverman, L.B.; Miller, T.L.; Barry, E.V.; Asselin, B.L.; Athale, U.; Clavell, L.A.; et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicintreated children with high-risk acute lymphoblastic leukaemia: Longterm follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010, 11, 950–961.
- Paiva, M.G.; Petrilli, A.S.; Moises, V.A.; Macedo, C.R.; Tanaka, C.; Campos, O. Cardioprotective effect of dexrazoxane during treatment with doxorubicin: A study using low-dose dobutamine stress echocardiography. Pediatr. Blood Cancer 2005, 45, 902–908.
- Asselin, B.L.; Devidas, M.; Chen, L.; Franco, V.I.; Pullen, J.; Borowitz, M.J.; Hutchison, R.E.; Ravindranath, Y.; Armenian, S.H.; Camitta, B.M.; et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed t-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: A report of the Children’s oncology group randomized trial pediatric oncology group 9404. J. Clin. Oncol. 2016, 34, 854–862.
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337.
- Ganatra, S.; Nohria, A.; Shah, S.; Groarke, J.D.; Sharma, A.; Venesy, D.; Patten, R.; Gunturu, K.; Zarwan, C.; Neilan, T.G.; et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: A consecutive case series. Cardio-Oncology 2019, 5, 1.
- Soultati, A.; Mountzios, G.; Avgerinou, C.; Papaxoinis, G.; Pectasides, D.; Dimopoulos, M.A.; Papadimitriou, C. Endothelial vascular toxicity from chemotherapeutic agents: Preclinical evidence and clinical implications. Cancer Treat. Rev. 2012, 38, 473–483.
- Mulrooney, D.A.; Blaes, A.H.; Duprez, D. Vascular injury in cancer survivors. J. Cardiovasc. Transl. Res. 2012, 5, 287–295.
- Ascensao, A.; Magalhaes, J.; Soares, J.M.; Ferreira, R.; Neuparth, M.J.; Marques, F.; Oliveira, P.J.; Duarte, J.A. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H722–H731.
- Tranchita, E.; Murri, A.; Grazioli, E.; Cerulli, C.; Emerenziani, G.P.; Ceci, R.; Caporossi, D.; Dimauro, I.; Parisi, A. The Beneficial Role of Physical Exercise on Anthracyclines Induced Cardiotoxicity in Breast Cancer Patients. Cancers 2022, 14, 2288.
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The health, eating, activity, and lifestyle (HEAL) study. Cancer 2003, 97, 1746–1757.
- Rock, C.L.; Flatt, S.W.; Newman, V.; Caan, B.J.; Haan, M.N.; Stefanick, M.L.; Faerber, S.; Pierce, J.P. Factors associated with weight gain in women after diagnosis of breast cancer. Women’s healthy eating and living study group. J. Am. Diet Assoc. 1999, 99, 1212–1221.
- Tham, E.B.; Haykowsky, M.J.; Chow, K.; Spavor, M.; Kaneko, S.; Khoo, N.S.; Pagano, J.J.; Mackie, A.S.; Thompson, R.B. Diffuse myocardial fibrosis by T1- mapping in children with subclinical anthracycline cardiotoxicity: Relationship to exercise capacity, cumulative dose and remodeling. J. Cardiovasc. Magn. Reson. 2013, 15, 48.
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426.
