Restorative and adhesive dentistry has witnessed extraordinary improvements after the innovations in contemporary adhesive materials. These new adhesive systems do not require any mechanical retention through features such as dovetails, grooves, sharp internal angles, and undercuts. For the success of modern restorative dentistry, these adhesive systems play a critical role, as sound tooth structure would be preserved using these newer systems. In addition, by using these contemporary and advanced adhesive systems, secondary caries due to microleakage may be reduced or eliminated.
- biomimetic
- bond degradation
- dental adhesive
- dentine-resin interface
- resin composites
1. Degradation of Adhesive Interface/Hybrid Layer
1.1. Degradation of Adhesive Resins
1.2. Degradation of Collagen
1.2.1. Matrix Metalloproteinase (MMP)
1.2.2. Cysteine Cathepsins
1.3. Incomplete Infiltration of the Resin Adhesives
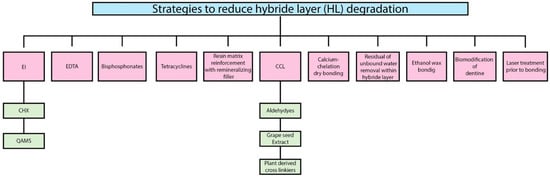
2. Strategies to Reduce Hybrid Layer (HL) Degradation
2.1. Enzymes Inhibitors
2.1.1. Chlorhexidine (CHX)
2.1.2. Quaternary Ammonium Methacrylates Compounds (QAMS)
2.2. Ethylene Diamine Tetra Acetic Acid (EDTA)
For decades, due to the chelating properties, ethylene-diamine tetra acetic acid (EDTA) has been used in endodontics. EDTA binds to Zn2+ ions from the catalytic site of the MMPs and removes the Ca2+ from the collagen matrices [49][50]. However, a long application time and the reversibility caused by water solubility are the main drawbacks [51].
2.3. Bisphosphonates
2.4. Tetracycline
2.5. Collagen Cross Linkers
-
Lastly, many different mechanisms have been involved in the inhibition of MMPs and CTs by these cross-linkers.
2.6. Residual or Unbound Water Removal within the Hybrid Layer
2.7. Calcium-Chelation Dry Bonding
2.8. Biomodification of Dentine
2.9. Ethanol Wet Bonding
2.10. Resin Matrix Reinforcement with Remineralizing Fillers
2.11. Laser Treatment Prior to Bonding
3. Biomimetic Remineralization
- (a)
-
The first approach cannot occur in demineralized dentine where apatite crystals are absent. In this type of remineralization, the remaining mineral crystals act as templates for the regrowth of apatite crystals [97].
- (b)
-
The second type of biomimetic remineralization involves incorporating polyanions (polyacrylic acid/polyaspartic acid) and apatite nucleation, resulting in biomimetic remineralization [97].
This entry is adapted from the peer-reviewed paper 10.3390/coatings12081094
References
- Kermanshahi, S.; Santerre, J.; Cvitkovitch, D.; Finer, Y. Biodegradation of resin-dentine interfaces increases bacterial microleakage. J. Dent. Res. 2010, 89, 996–1001.
- Hashimoto, M. A Review—Micromorphological evidence of degradation in resin-dentine bonds and potential preventional solutions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 268–280.
- Hosaka, K.; Nishitani, Y.; Tagami, J.; Yoshiyama, M.; Brackett, W.W.; Agee, K.A.; Tay, F.R.; Pashley, D.H. Durability of resin-dentin bonds to water-vs. ethanol-saturated dentin. J. Dent. Res. 2009, 88, 146–151.
- Manappallil, J.J. Basic Dental Materials; JP Medical Ltd.: Clayton, Panama, 2015.
- Loguercio, A.D.; Moura, S.K.; Pellizzaro, A.; Dal-Bianco, K.; Patzlaff, R.T.; Grande, R.H.M. Durability of enamel bonding using two-step self-etch systems on ground and unground enamel. Oper. Dent. 2008, 33, 79–88.
- Malacarne, J.; Carvalho, R.M.; Mario, F.; Svizero, N.; Pashley, D.H.; Tay, F.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. J. 2006, 22, 973–980.
- Loguercio, A.D.; Bittencourt, D.D.; Baratieri, L.N.; Reis, A. A 36-month evaluation of self-etch and etch-and-rinse adhesives in noncarious cervical lesions. J. Am. Dent. Assoc. 2007, 138, 507–514.
- Yiu, C.K.; Pashley, E.L.; Hiraishi, N.; King, N.M.; Goracci, C.; Ferrari, M.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials 2005, 26, 6863–6872.
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J. Limitations in bonding to dentine and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968.
- Ye, Q.; Park, J.; Topp, E.; Wang, Y.; Misra, A.; Spencer, P. In vitro performance of nano-heterogeneous dentine adhesive. J. Dent. Res. 2008, 87, 829–833.
- Wang, Y.; Spencer, P.; Yao, X.; Ye, Q. Effect of coinitiator and water on the photoreactivity and photopolymerization of HEMA/camphoquinone-based reactant mixtures. J. Biomed. Mater. Res. A 2006, 78, 721–728.
- Ilie, N.; Hickel, R. Can CQ be completely replaced by alternative initiators in dental adhesives? Dent. Mater. J. 2008, 27, 221–228.
- Cadenaro, M.; Antoniolli, F.; Codan, B.; Agee, K.; Tay, F.R.; Dorigo, E.D.S. Influence of different initiators on the degree of conversion of experimental adhesive blends in relation to their hydrophilicity and solvent content. Dent. Mater. J. 2010, 26, 288–294.
- Amaral, F.L.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–353.
- Shokati, B.; Tam, L.E.; Santerre, J.P.; Finer, Y. Effect of salivary esterase on the integrity and fracture toughness of the dentine-resin interface. J. Biomed. Mater. Res, B. Appl. Biomater. 2010, 94, 230–237.
- Kostoryz, E.L.; Dharmala, K.; Ye, Q.; Wang, Y.; Huber, J.; Park, J.G. Enzymatic biodegradation of HEMA/bisGMA adhesives formulated with different water content. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 394–401.
- Bahudhanapati, H. In Search of MMP Specific Inhibitors: Protein Engineering of TIMPs; Florida Atlantic University: Boca Raton, FL, USA, 2009.
- Shimada, Y.; Ichinose, S.; Sadr, A.; Burrow, M.; Tagami, J. Localization of matrix metalloproteinases (MMPs-2; 8; 9 and 20) in normal and carious dentine. Aust. Dent. J. 2009, 54, 347–354.
- Tersariol, I.L.; Geraldeli, S.; Minciotti, C.L.; Nascimento, F.D.; Pääkkönen, V.; Martins, M.T.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Salo, T.; et al. Cysteine cathepsins in human dentine-pulp complex. J. Endod. 2010, 36, 475–481.
- Nascimento, F.; Minciotti, C.; Geraldeli, S.; Carrilho, M.; Pashley, D.H.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjäderhane, L.; Tersariol, I.L.S. Cysteine cathepsins in human carious dentine. J. Dent. Res. 2011, 90, 506–511.
- Mahalaxmi, S.; Madhubala, M.; Jayaraman, M.; Sathyakumar, S. Evaluation of matrix metalloproteinase and cysteine cathepsin activity in dentine hybrid layer by gelatin zymography. Indian J. Dent. Res. 2016, 27, 652.
- Li, Z.; Kienetz, M.; Cherney, M.M.; James, M.N.; Brömme, D. The crystal and molecular structures of a cathepsin K; chondroitin sulfate complex. J. Mol. Biol. 2008, 383, 78–91.
- Vidal, C.; Tjäderhane, L.; Scaffa, P.; Tersariol, I.; Pashley, D.; Nader, H. Abundance of MMPs and cysteine cathepsins in caries-affected dentine. J. Dent. Res. 2014, 93, 269–274.
- Scaffa, P.M.; Breschi, L.; Mazzoni, A.; Vidal, C.D.; Curci, R.; Apolonio, F.; Gobbi, P.; Pashley, D.; Tjäderhane, L.; dos Santos Tersariol, I.L.; et al. Co-distribution of cysteine cathepsins and matrix metalloproteases in human dentine. Arch. Oral. Biol. 2017, 74, 101–107.
- Baldión, P.A.; Cortés, C.J. Mathematical models of polymer-dentine physicochemical interactions and their biological effects. Sci. J. Rev. 2016, 5, 319–330.
- Kim, Y.K.; Gu, L.-S.; Bryan, T.E.; Kim, J.R.; Chen, L.; Liu, Y. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium; phosphate and hydroxyl ions. Biomaterials 2010, 31, 6618–6627.
- Jee, S.E.; Zhou, J.; Tan, J.; Breschi, L.; Tay, F.R.; Grégoire, G. Investigation of ethanol infiltration into demineralized dentine collagen fibrils using molecular dynamics simulations. Acta Biomate 2016, 36, 175–185.
- Bertassoni, L.E. Dentine on the nanoscale; Hierarchical organization; mechanical behavior and bioinspired engineering. Dent. Mater. J. 2017, 33, 637–649.
- Fung, D.T.; Wang, V.M.; Laudier, D.M.; Shine, J.H.; Basta-Pljakic, J.; Jepsen, K.J. Subrupture tendon fatigue damage. J. Orthop. Res. 2009, 27, 264–273.
- Carrilho, M.R.; Carvalho, R.M.; Sousa, E.N.; Nicolau, J.; Breschi, L.; Mazzoni, A. Substantivity of chlorhexidine to human dentine. Dent. Mater. J. 2010, 26, 779–785.
- Carrilho, M.; Carvalho, R.M.; De Goes, M.; Di Hipolito, V.; Geraldeli, S.; Tay, F.R. Chlorhexidine preserves dentine bond in vitro. J. Dent. Res. 2007, 86, 90–94.
- Breschi, L.; Mazzoni, A.; Nato, F.; Carrilho, M.; Visintini, E.; Tjäderhane, L.; Ruggeri, A., Jr.; Tay, F.R.; Dorigo, E.D.; Pashley, D.H. Chlorhexidine stabilizes the adhesive interface; a 2-year in vitro study. Dent. Mater. J. 2010, 26, 320–325.
- Loguercio, A.D.; Hass, V.; Gutierrez, M.F.; Luque-Martinez, I.V.; Szezs, A.; Stanislawczuk, R. Five-year effects of chlorhexidine on the in vitro durability of resin/dentine interfaces. J. Adhes. Dent. 2016, 18, 35–42.
- Zheng, P.; Zaruba, M.; Attin, T.; Wiegand, A. Effect of different matrix metalloproteinase inhibitors on microtensile bond strength of an etch-and-rinse and a self-etching adhesive to dentine. Oper. Dent. J. 2015, 40, 80–86.
- Hebling, J.; Pashley, D.H.; Tjäderhane, L.; Tay, F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005, 84, 741–746.
- Moharam, L.-M.; Salem, H.-N.; Elgamily, H.-M. The effect of incorporating different concentrations of chlorhexidine digluconate on the degree of conversion of an experimental adhesive resin. J. Clinc. Exp. 2018, 10, e371.
- Cadenaro, M.; Pashley, D.H.; Marchesi, G.; Carrilho, M.; Antoniolli, F.; Mazzoni. Influence of chlorhexidine on the degree of conversion and E-modulus of experimental adhesive blends. Dent. Mater. J. 2009, 25, 1269–1274.
- Stanislawczuk, R.; Pereira, F.; Muñoz, M.A.; Luque, I.; Farago, P.V.; Reis, A. Effects of chlorhexidine-containing adhesives on the durability of resin–dentine interfaces. J. Dent. 2014, 42, 39–47.
- Da Silva, E.M.; de Sá Rodrigues, C.U.F.; de Oliveira Matos, M.P.; de Carvalho, T.R.; dos Santos, G.B.; Amaral, C.M. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors; Physicochemical characterization and resin-dentine bonding stability. J. Dent. 2015, 43, 1491–1497.
- Tezvergil-Mutluay, A.; Mutluay, M.M.; Gu, L.S.; Zhang, K.; Agee, K.A.; Carvalho, R.M.; Manso, A.; Carrilho, M.; Tay, F.R.; Breschi, L.; et al. The anti-MMP activity of benzalkonium chloride. J. Dent. 2011, 39, 57–64.
- Sabatini, C.; Patel, S.K. Matrix metalloproteinase inhibitory properties of benzalkonium chloride stabilizes adhesive interfaces. Eur. J. Oral. Sci. 2013, 121, 610–616.
- Sabatini, C.; Ortiz, P.A.; Pashley, D.H. Preservation of resin–dentine interfaces treated with benzalkonium chloride adhesive blends. Eur. J. Oral Sci. 2015, 123, 108–115.
- Hoshika, T.; Nishitani, Y.; Yoshiyama, M.; Key, W.O., III; Brantley, W.; Agee, K.A.; Breschi, L.; Cadenaro, M.; Tay, F.R.; Rueggeberg, F.; et al. Effects of quaternary ammonium-methacrylates on the mechanical properties of unfilled resins. Dent. Mater. J. 2014, 30, 1213–1223.
- Tezvergil-Mutluay, A.; Agee, K.A.; Mazzoni, A.; Carvalho, R.M.; Carrilho, M.; Tersariol, I.L. Can quaternary ammonium methacrylates inhibit matrix MMPs and cathepsins? Dent. Mater. J. 2015, 31, e25–e32.
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth; adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296.
- Imazato, S.; Tay, F.R.; Kaneshiro, A.V.; Takahashi, Y.; Ebisu, S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater. J. 2007, 23, 170–176.
- Pashley, D.H.; Tay, F.R.; Imazato, S. How to increase the durability of resin-dentine bonds. Compend. Contin. Educ. Dent. 2011, 32, 60–64.
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. J. 2012, 28, 219–228.
- Thompson, J.M.; Agee, K.; Sidow, S.J.; McNally, K.; Lindsey, K.; Borke, J. Inhibition of endogenous dentine matrix metalloproteinases by ethylenediaminetetraacetic acid. J. Endod. 2012, 38, 62–65.
- Osorio, R.; Erhardt, M.C.; Pimenta, L.A.; Osorio, E.; Toledano, M. EDTA treatment improves resin-dentin bonds’ resistance to degradation. J. Dent. Res. 2005, 84, 736–740.
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Tjäderhane, L.; Mazzoni, A. Host-derived loss of dentine matrix stiffness associated with solubilization of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 373–380.
- Heikkilä, P.; Teronen, O.; Moilanen, M.; Konttinen, Y.T.; Hanemaaijer, R.; Laitinen, M.; Maisi, P.; van der Pluijm, G.; Bartlett, J.D.; Salo, T.; et al. Bisphosphonates inhibit stromelysin-1 (MMP-3); matrix metalloelastase (MMP-12); collagenase-3 (MMP-13) and enamelysin (MMP-20); but not urokinase-type plasminogen activator; and diminish invasion and migration of human malignant and endothelial cell lines. Anti-Cancer Drugs 2002, 13, 245–254.
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Tay, F.R.; Pashley, D.H. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentine. Acta Biomater. 2010, 6, 4136–4142.
- Gu, L.-S.; Kim, Y.K.; Liu, Y.; Takahashi, K.; Arun, S.; Wimmer, C.E. Immobilization of a phosphonated analog of matrix phosphoproteins within cross-linked collagen as a templating mechanism for biomimetic mineralization. Acta Biomater. 2011, 7, 268–277.
- Mazzoni, A.; Mannello, F.; Tay, F.R.; Tonti, G.A.; Papa, S.; Mazzotti, G.; Di Lenarda, R.; Pashley, D.H.; Breschi, L. Zymographic analysis and characterization of MMP-2 and-9 forms in human sound dentin. J. Dent. Res. 2007, 86, 436–440.
- Sorsa, T.; Tjäderhane, L.; Konttinen, Y.T.; Lauhio, A.; Salo, T.; Lee, H.M. Matrix metalloproteinases; contribution to pathogenesis; diagnosis and treatment of periodontal inflammation. Ann. Med. 2006, 38, 306–321.
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.; Pashley, D.H.; Tay, F. Zinc reduces collagen degradation in demineralized human dentine explants. J. Dent. 2011, 39, 148–153.
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006, 85, 22–32.
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases; fold and function of their catalytic domains. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 20–28.
- Bedran-Russo, A.K.; Pauli, G.F.; Chen, S.N.; McAlpine, J.; Castellan, C.S.; Phansalkar, R.S.; Aguiar, T.R.; Vidal, C.M.; Napotilano, J.G.; Nam, J.W.; et al. Dentine biomodification; strategies; renewable resources and clinical applications. Dent. Mater. J. 2014, 30, 62–76.
- Xu, C.; Wang, Y. Cross-linked demineralized dentine maintains its mechanical stability when challenged by bacterial collagenase. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 242–248.
- Naso, F.; Gandaglia, A.; Bottio, T.; Tarzia, V.; Nottle, M.B.; d’Apice, A.J.; Cowan, P.J.; Cozzi, E.; Galli, C.; Lagutina, I.; et al. First quantification of alpha-G al epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation 2013, 20, 252–261.
- Pashley, D.H.; Tay, F.R.; Carvalho, R.M.; Rueggeberg, F.A.; Agee, K.A.; Carrilho, M. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentine matrix and solvated resins using a macromodel of the hybrid layer. Am. J. Dent. 2007, 20, 7.
- Tay, F.; Pashley, D.H.; Kapur, R.; Carrilho, M.; Hur, Y.; Garrett, L. Bonding BisGMA to dentine—A proof of concept for hydrophobic dentine bonding. J. Dent. Res. 2007, 86, 1034–1039.
- Ayar, M.K. A review of ethanol wet-bonding; Principles and techniques. Eur. Dent. J. 2016, 10, 155.
- Hiraishi, N.; Nishiyama, N.; Ikemura, K.; Yau, J.; King, N.; Tagami, J. Water concentration in self-etching primers affects their aggressiveness and bonding efficacy to dentine. J. Dent. Res. 2005, 84, 653–658.
- Shin, T.P.; Yao, X.; Huenergardt, R.; Walker, M.P.; Wang, Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentine using ethanol wet bonding technique. Dent. Mater. J. 2009, 25, 1050–1057.
- Agee, K.A.; Prakki, A.; Abu-Haimed, T.; Naguib, G.H.; Nawareg, M.A.; Tezvergil-Mutluay, A. Water distribution in dentine matrices; bound, vs. unbound water. Dent. Mater. J. 2015, 31, 205–216.
- Chiba, A.; Zhou, J.; Nakajima, M.; Tan, J.; Tagami, J.; Scheffel, D. The effects of ethanol on the size-exclusion characteristics of type I dentine collagen to adhesive resin monomers. Acta Biomater. 2016, 33, 235–241.
- Takahashi, M.; Nakajima, M.; Tagami, J.; Scheffel, D.; Carvalho, R.; Mazzoni, A. The importance of size-exclusion characteristics of type I collagen in bonding to dentine matrices. Acta Biomater. 2013, 9, 9522–9528.
- Mai, S.; Wei, C.-C.; Gu, L.-S.; Tian, F.-C.; Arola, D.D.; Chen, J.-H. Extrafibrillar collagen demineralization-based chelate-and-rinse technique bridges the gap between wet and dry dentine bonding. Acta Biomater. 2017, 57, 435–448.
- Milia, E.; Cumbo, E.; Cardoso, J.A.; Gallina, G. Current dental adhesives systems. A narrative review. Curr. Pharma.Des. 2012, 18, 5542–5552.
- De Oliveira Carrilho, M.R.; Tay, F.R.; Pashley, D.H.; Tjäderhane, L.; Carvalho, R.M. Mechanical stability of resin–dentine bond components. Dent. Mater. J. 2005, 21, 232–241.
- Reis, A.F.; Carrilho, M.R.; Ghaname, E.; Pereira, P.N.; Giannini, M.; Nikaido, T. Effects of water-storage on the physical and ultramorphological features of adhesives and primer/adhesive mixtures. Dent. Mater. J. 2010, 29, 1011170090.
- Brackett, M.G.; Li, N.; Brackett, W.W.; Sword, R.J.; Qi, Y.; Niu, L. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J. Dent. 2011, 39, 238–248.
- Celik, C.; Ozgunaltay, G.; Dayangaç, B. Bond strength of different adhesive systems to dental hard tissues. Oper. Dent. 2007, 32, 166–172.
- Sadek, F.; Braga, R.; Muench, A.; Liu, Y.; Pashley, D.H.; Tay, F. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 2010, 89, 1499–1504.
- Sadek, F.T.; Castellan, C.S.; Braga, R.R.; Mai, S.; Tjäderhane, L.; Pashley, D.H. One-year stability of resin–dentine bonds created with a hydrophobic ethanol-wet bonding technique. Dent. Mater. J. 2010, 26, 380–386.
- Gutiérrez, M.; Malaquias, P.; Matos, T.; Szesz, A.; Souza, S.; Bermudez, J. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent. Mater. J. 2017, 33, 309–320.
- Sun, J.; Petersen, E.J.; Watson, S.S.; Sims, C.M.; Kassman, A.; Frukhtbeyn, S.; Skrtic, D.; Ok, M.T.; Jacobs, D.S.; Reipa, V.; et al. Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives. Acta Biomater. 2017, 53, 585–597.
- Torres-Mendez, F.; Martinez-Castanon, G.-A.; Torres-Gallegos, I.; Zavala-Alonso, N.-V.; Patino-Marin, N.; Nino-Martinez, N. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent. Mater. 2017, 36, 266–274.
- Barcellos, D.C.; Fonseca, B.M.; Pucci, C.R.; das Neves Cavalcanti, B.; Persici, E.D.S.; de Paiva Gonçalves, S.E. Zn-doped etch-and-rinse model dentine adhesives; Dentine bond integrity; biocompatibility; and properties. Dent. Mater. 2016, 32, 940–950.
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.-D. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546.
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. J. 2016, 32, 41–53.
- Agarwal, U.S.; Nisal, A.; Joseph, R. PET-SWNT nanocomposites through ultrasound assisted dissolution-evaporation. Eur. Polym. J. 2007, 43, 2279–2285.
- Alkatheeri, M.S.; Palasuk, J.; Eckert, G.J.; Platt, J.A.; Bottino, M.C. Halloysite nanotube incorporation into adhesive systems—Effect on bond strength to human dentine. Clinic. Oral. Investig. 2015, 19, 1905–1912.
- Feitosa, S.A.; Münchow, E.A.; Al-Zain, A.O.; Kamocki, K.; Platt, J.A.; Bottino, M.C. Synthesis and characterization of novel halloysite-incorporated adhesive resins. J. Dent. 2015, 43, 1316–1322.
- Feitosa, S.; Palasuk, J.; Kamocki, K.; Geraldeli, S.; Gregory, R.; Platt, J. Doxycycline-encapsulated nanotube-modified dentine adhesives. J. Dent. Res. 2014, 93, 1270–1276.
- Leitune, V.C.B.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M.W. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327.
- Curylofo, F.A.; Messias, D.C.F.; Silva-Sousa, Y.T.C.; Souza-Gabriel, A.E. Bond strength of restorative material to dentine submitted to bleaching and Er; YAG laser post-treatment. Photomed Laser Surg. 2014, 32, 495–499.
- Kasraei, S.; Yarmohammadi, E.; Ghazizadeh, M.V. Microshear bond strength of OptiBond all-in-one self-adhesive agent to Er; YAG laser treated enamel after thermocycling and water storage. Lasers. Med. Sci. 2016, 7, 152.
- Han, G.J.; Kim, J.H.; Chung, S.N.; Chun, B.H.; Kim, C.K.; Seo, D.G. Effects of non-thermal atmospheric pressure pulsed plasma on the adhesion and durability of resin composite to dentine. Eur. J. Oral Sci. 2014, 122, 417–423.
- de Abreu, J.L.B.; Prado, M.; Simão, R.A.; da Silva, E.M.; Dias, K.R.H.C. Effect of non-thermal argon plasma on bond strength of a self-etch adhesive system to NaOCl-treated dentine. Braz. Dent. 2016, 27, 446–451.
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34.
- Bertassoni, L.E.; Habelitz, S.; Pugach, M.; Soares, P.C.; Marshall, S.J.; Marshall Jr, G.W. Evaluation of surface structural and mechanical changes following remineralization of dentine. Scanning Microsc. 2010, 32, 312–319.
- He, L.; Hao, Y.; Zhen, L.; Liu, H.; Shao, M.; Xu, X.; Liang, K.; Gao, Y.; Yuan, H.; Li, J.; et al. Biomineralization of dentine. J. Struct. Biol. 2019, 207, 115–122.
- Rokidi, S.; Koutsoukos, P.G. Crystal growth of calcium phosphates from aqueous solutions in the presence of strontium. Chem. Eng. Sci. 2012, 77, 157–164.
