Breast cancer (BC) is the main type of cancer in women and the second most frequent cancer worldwide. The conventional treatment includes surgery, chemotherapy, hormonal therapy, and immunotherapy. This immunotherapy is based on administering monoclonal therapeutic antibodies (passive) or vaccines (active) with therapeutic purposes. Tumor antigens are classified as tumor-associated antigens (TAAs) and tumor-specific antigens (TSA). New TAAs were proposed for the formulation of peptide-based vaccines, including MUC-1 (mucin-1), FRα (folate receptor alpha), members of the MAGE A family (melanoma-associated antigen), and EGFR (epidermal growth factor receptor).
- peptide-based vaccines
- breast cancer
- therapeutic
1. Peptide-Based Vaccines for Breast Cancer
| Vaccine | Description | Clinical Phase | Evidence |
|---|---|---|---|
| NeuVax™ Peptide derived from HER2. |
Breast cancer with low or moderate expression of HER2. GM-CSF and water. | III | Register: NCT01479244 [13] |
| GP2 Peptide derived from HER2. |
Breast cancer HLA-A2+ with positive lymph nodes in tumors with positive HER2 expression. | II | [10][14] |
| AE37 Peptide derived from HER2. |
Breast cancer with positive and negative lymph nodes, with positive expression of HER2. | II | [10] |
| KRM-19 Mixed vaccine of 19 peptides derived from multiple AATs. |
Metastatic triple-negative breast cancer with resistance to the conventional treatment. | II | Register: UMIN000014616 [6] |
| Nelipepimut-S peptide + GM-CSF + trastuzumab. | Breast cancer with low expression of HER2. | II | [15] |
| HLA-matched personalized peptide vaccine. | Recurrent metastatic breast cancer. | II | Register: UMIN000001844 [16] |
| P10s-PADRE | Triple-negative breast cancer (TNBC) in stages I, II, or III. | I/II | Register: NCT02938442 |
| Multipeptide MUC1/ErbB2/CEA | high-risk disease-free ovarian and breast cancer after completion of standard therapies. | I/II | [7] |
| FRα multi-epitope |
Vaccine + cyclophosphamide + sargramostim in treating patients with stage II-III breast cancer. | II | Register: NCT03012100 |
| MAG-TN3+ AS15 | Breast neoplasms. | I | Register: NCT02364492 [4][17] |
| Mimotope P10s-PADRE/MONTANIDE ISA 51 VG | Peptide mimotope-based vaccine of tumor-associated carbohydrate antigens in patients with stage IV breast cancer. | I | Register: NCT01390064 [8] |
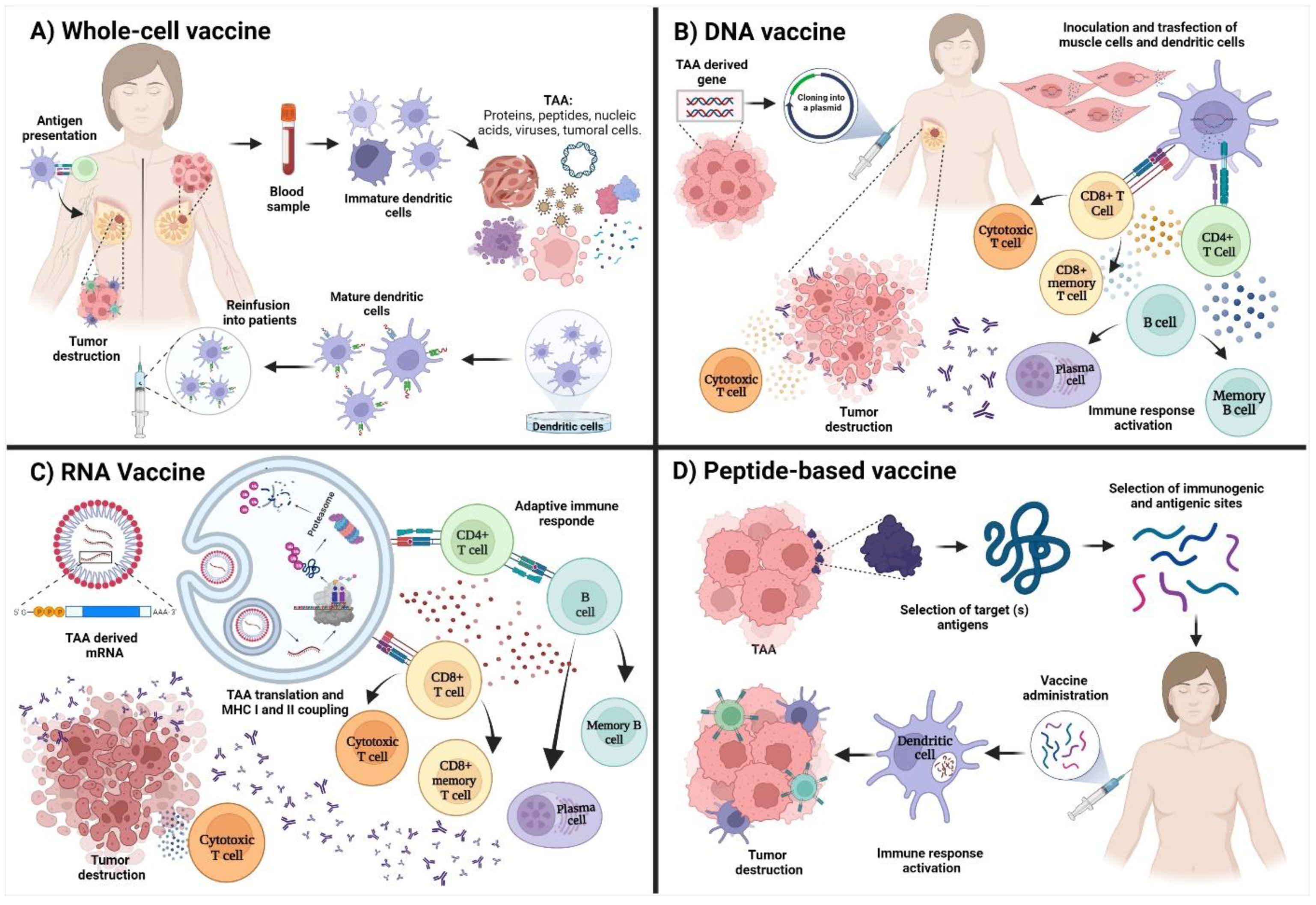
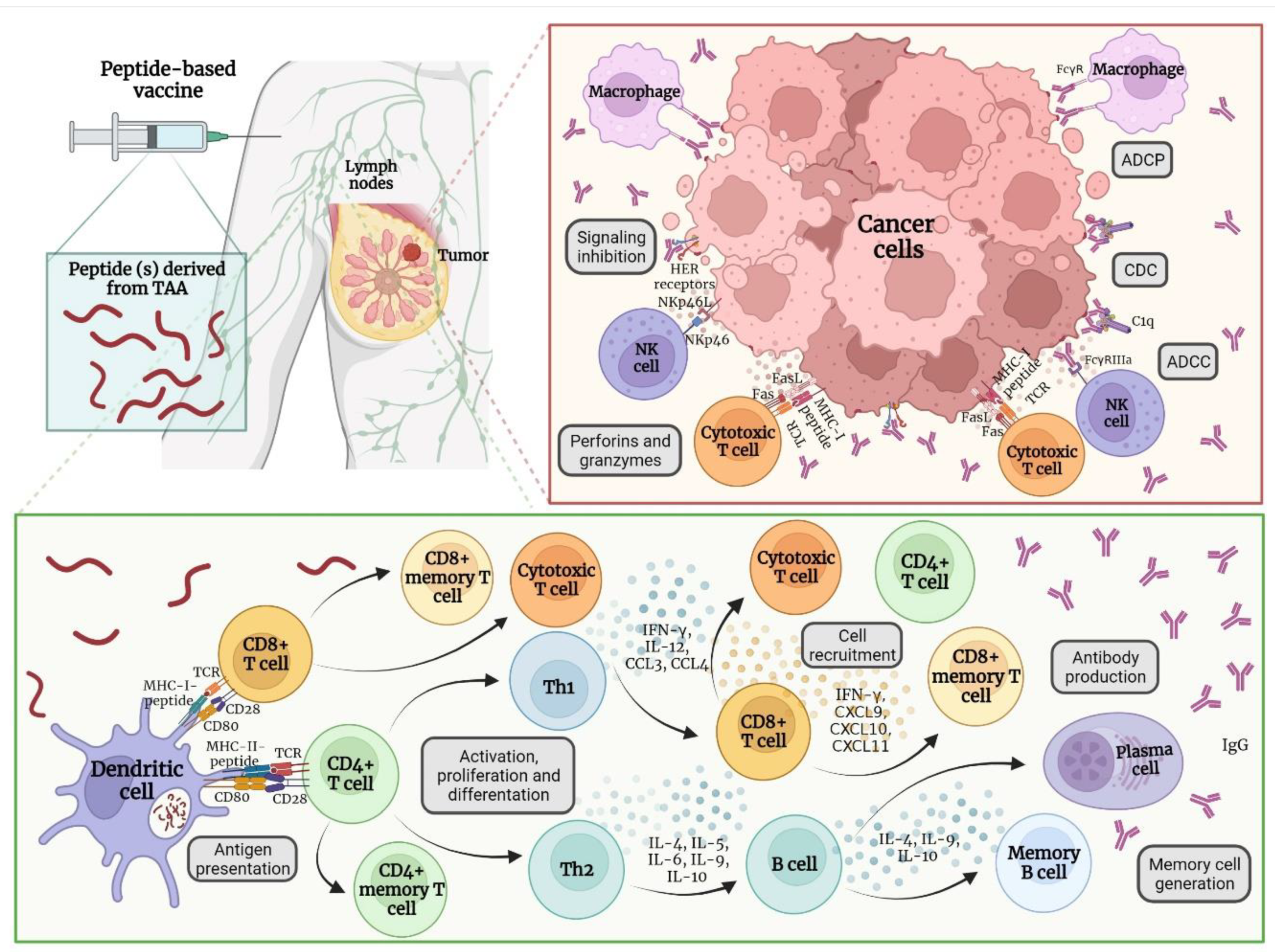
2. Immune Response after Vaccine Administration
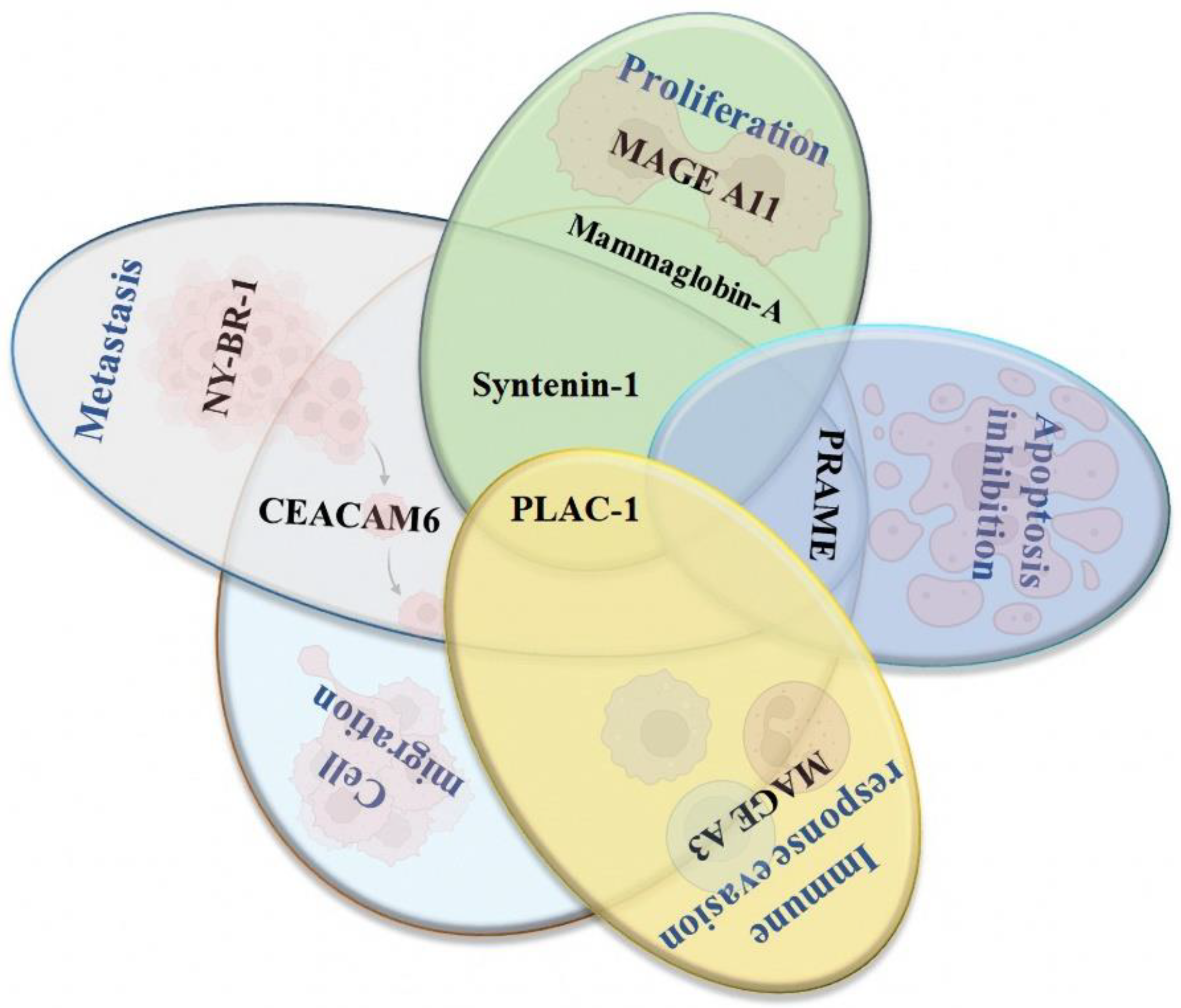
3. New Potential Therapeutic Targets for Developing Peptide-Based Vaccines for Breast Cancer
3.1. Syntenin-1
3.2. PLAC-1
3.3. Mammaglobin-α
3.4. NY-BR-1
3.5. PRAME
3.6. MAGE A3 and A11
3.7. CEACAM6
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10081249
References
- Geng, F.; Bao, X.; Dong, L.; Guo, Q.-Q.; Guo, J.; Xie, Y.; Zhou, Y.; Yu, B.; Wu, H.; Wu, J.-X.; et al. Doxorubicin pretreatment enhances FAPα/survivin co-targeting DNA vaccine anti-tumor activity primarily through decreasing peripheral MDSCs in the 4T1 murine breast cancer model. Oncoimmunology 2020, 9, 1747350.
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536.
- Dillon, P.M.; Petroni, G.R.; Smolkin, M.E.; Brenin, D.R.; Chianese-Bullock, K.A.; Smith, K.T.; Olson, W.C.; Fanous, I.S.; Nail, C.J.; Brenin, C.M.; et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 2017, 5, 92.
- Rosenbaum, P.; Artaud, C.; Bay, S.; Ganneau, C.; Campone, M.; Delaloge, S.; Gourmelon, C.; Loirat, D.; Medioni, J.; Pein, F.; et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol. Immunother. 2020, 69, 703–716.
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3014–3025.
- Toh, U.; Sakurai, S.; Saku, S.; Takao, Y.; Okabe, M.; Iwakuma, N.; Shichijo, S.; Yamada, A.; Itoh, K.; Akagi, Y. Early phase II study of mixed 19-peptide vaccine monotherapy for refractory triple-negative breast cancer. Cancer Sci. 2020, 111, 2760–2769.
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378.
- Hutchins, L.F.; Makhoul, I.; Emanuel, P.D.; Pennisi, A.; Siegel, E.R.; Jousheghany, F.; Guo, X.; Pashov, A.D.; Monzavi-Karbassi, B.; Kieber-Emmons, T. Targeting tumor-associated carbohydrate antigens: A phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget 2017, 8, 99161–99178.
- Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Gates, J.D.; Mittendorf, E.A.; Ponniah, S.; Peoples, G.E. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer 2010, 116, 292–301.
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 2016, 7, 66192–66201.
- Peoples, G.E.; Gurney, J.M.; Hueman, M.T.; Woll, M.M.; Ryan, G.B.; Storrer, C.E.; Fisher, C.; Shriver, C.D.; Ioannides, C.G.; Ponniah, S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7536–7545.
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742.
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4248–4254.
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401.
- Clifton, G.T.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Hale, D.F.; Herbert, G.S.; Litton, J.K.; Murthy, R.K.; Lukas, J.; Peoples, G.E.; et al. Initial safety analysis of a randomized phase II trial of nelipepimut-S + GM-CSF and trastuzumab compared to trastuzumab alone to prevent recurrence in breast cancer patients with HER2 low-expressing tumors. Clin. Immunol. 2019, 201, 48–54.
- Takahashi, R.; Toh, U.; Iwakuma, N.; Takenaka, M.; Otsuka, H.; Furukawa, M.; Fujii, T.; Seki, N.; Kawahara, A.; Kage, M.; et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res. 2014, 16, R70.
- Laubreton, D.; Bay, S.; Sedlik, C.; Artaud, C.; Ganneau, C.; Dériaud, E.; Viel, S.; Puaux, A.-L.; Amigorena, S.; Gérard, C.; et al. The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity. Cancer Immunol. Immunother. 2016, 65, 315–325.
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R.; et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2021, 266, 120431.
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The Peptide Vaccine of the Future. Mol. Cell Proteomics. 2021, 20, 100022.
- Owen, J.A.; Punt, J.; Stranford, S.A.; Jones, P.P.; Kuby, J. Kuby Inmunología: Séptima Edición, 7th ed.; McGraw Hill Educacion: Mexico City, México, 2014.
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776.
- Caballero, O.L.; Shousha, S.; Zhao, Q.; Simpson, A.J.G.; Coombes, R.C.; Neville, A.M. Expression of Cancer/Testis genes in ductal carcinoma in situ and benign lesions of the breast. Oncoscience 2014, 1, 14–20.
- Dyrskjøt, L.; Zieger, K.; Kissow Lildal, T.; Reinert, T.; Gruselle, O.; Coche, T.; Borre, M.; Ørntoft, T.F. Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br. J. Cancer 2012, 107, 116–122.
- Janelle, V.; Rulleau, C.; Del Testa, S.; Carli, C.; Delisle, J.S. T-Cell Immunotherapies Targeting Histocompatibility and Tumor Antigens in Hematological Malignancies. Front. Immunol. 2020, 11, 276.
- Das, S.K.; Bhutia, S.K.; Azab, B.; Kegelman, T.P.; Peachy, L.; Santhekadur, P.K.; Dasgupta, S.; Dash, R.; Dent, P.; Grant, S.; et al. MDA-9/Syntenin and IGFBP-2 Promote Angiogenesis in Human Melanoma. Cancer Res. 2013, 73, 844–854.
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 2016, 7, 80175–80189.
- Liu, J.; Yang, Y.; Wang, H.; Wang, B.; Zhao, K.; Jiang, W.; Bai, W.; Liu, J.; Yin, J. Syntenin1/MDA-9 (SDCBP) induces immune evasion in triple-negative breast cancer by upregulating PD-L1. Breast Cancer Res. Treat. 2018, 171, 345–357.
- Kashyap, R.; Roucourt, B.; Lembo, F.; Fares, J.; Carcavilla, A.M.; Restouin, A.; Zimmermann, P.; Ghossoub, R. Syntenin controls migration, growth, proliferation, and cell cycle progression in cancer cells. Front. Pharmacol. 2015, 6, 241.
- Yuan, H.; Wang, X.; Shi, C.; Jin, L.; Hu, J.; Zhang, A.; Li, J.; Vijayendra, N.; Doodala, V.; Weiss, S.; et al. Plac1 Is a Key Regulator of the Inflammatory Response and Immune Tolerance In Mammary Tumorigenesis. Sci. Rep. 2018, 8, 5717.
- Li, Y.; Chu, J.; Li, J.; Feng, W.; Yang, F.; Wang, Y.; Zhang, Y.; Sun, C.; Yang, M.; Vasilatos, S.N.; et al. Cancer/testis antigen-Plac1 promotes invasion and metastasis of breast cancer through Furin/NICD/PTEN signaling pathway. Mol. Oncol. 2018, 12, 1233–1248.
- Roldán, D.B.; Grimmler, M.; Hartmann, C.; Hubich-Rau, S.; Beißert, T.; Paret, C.; Cagna, G.; Rohde, C.; Wöll, S.; Koslowski, M.; et al. PLAC1 is essential for FGF7/FGFRIIIb-induced Akt-mediated cancer cell proliferation. Oncotarget 2020, 11, 1862–1875.
- Liu, W.; Zhai, M.; Wu, Z.; Qi, Y.; Wu, Y.; Dai, C.; Sun, M.; Li, L.; Gao, Y. Identification of a novel HLA-A2-restricted cytotoxic T lymphocyte epitope from cancer-testis antigen PLAC1 in breast cancer. Amino Acids 2012, 42, 2257–2265.
- Oloomi, M.; Moazzezy, N.; Bouzari, S. Comparing blood versus tissue-based biomarkers expression in breast cancer patients. Heliyon 2020, 6, e03728.
- Wang, Z.; Spaulding, B.; Sienko, A.; Liang, Y.; Li, H.; Nielsen, G.; Gong, G.Y.; Ro, J.Y.; Zhai, Q.J. Mammaglobin, a valuable diagnostic marker for metastatic breast carcinoma. Int. J. Clin. Exp. Pathol. 2009, 2, 384–389.
- Hu, Y.; Liu, P.; Wu, D.; Jiang, Y. Prognostic role of plasma mammaglobin A expression in breast carcinoma patients: A meta-analysis. OncoTargets Ther. 2018, 11, 3245–3255.
- Kim, S.W.; Goedegebuure, P.; Gillanders, W.E. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology 2016, 5, e1069940.
- Galvis Jiménez, J.M.; Curtidor, H.; Patarroyo, M.A.; Monterrey, P.; Ramírez Clavijo, S.R. Mammaglobin peptide as a novel biomarker for breast cancer detection. Cancer Biol. Ther. 2013, 14, 327–332.
- Kosaloglu, Z.; Bitzer, J.; Halama, N.; Huang, Z.; Zapatka, M.; Schneeweiss, A.; Jäger, D.; Zörnig, I. In silico SNP analysis of the breast cancer antigen NY-BR-1. BMC Cancer 2016, 16, 901.
- Theurillat, J.-P.; Ingold, F.; Frei, C.; Zippelius, A.; Varga, Z.; Seifert, B.; Chen, Y.-T.; Jäger, D.; Knuth, A.; Moch, H. NY-ESO-1 protein expression in primary breast carcinoma and metastases: Correlation with CD8+ T-cell and CD79a+ plasmacytic/B-cell infiltration. Int. J. Cancer. 2007, 120, 2411–2417.
- Balafoutas, D.; Hausen, A.Z.; Mayer, S.; Hirschfeld, M.; Jaeger, M.; Denschlag, D.; Gitsch, G.; Jungbluth, A.; Stickeler, E. Cancer testis antigens and NY-BR-1 expression in primary breast cancer: Prognostic and therapeutic implications. BMC Cancer 2013, 13, 271.
- Gardyan, A.; Osen, W.; Zörnig, I.; Podola, L.; Agarwal, M.; Aulmann, S.; Ruggiero, E.; Schmidt, M.; Halama, N.; Leuchs, B.; et al. Identification of NY-BR-1-specific CD4(+) T cell epitopes using HLA-transgenic mice. Int. J. Cancer 2015, 136, 2588–2597.
- Epping, M.T.; Bernards, R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006, 66, 10639–10642.
- Epping, M.T.; Hart, A.A.M.; Glas, A.M.; Krijgsman, O.; Bernards, R. PRAME expression and clinical outcome of breast cancer. Br. J. Cancer 2008, 99, 398–403.
- Li, J.; Yin, J.; Zhong, J.; Yang, Z.; Tang, A.; Li, S. Clinicopathological and Prognostic Significance of PRAME Overexpression in Human Cancer: A Meta-Analysis. BioMed Res. Int. 2020, 2020, 8828579.
- Al-Khadairi, G.; Naik, A.; Thomas, R.; Al-Sulaiti, B.; Rizly, S.; Decock, J. PRAME promotes epithelial-to-mesenchymal transition in triple negative breast cancer. J. Transl. Med. 2019, 17, 9.
- Taheri Anganeh, M.; Savardashtaki, A.; Vafadar, A.; Movahedpour, A.; Shabaninejad, Z.; Maleksabet, A.; Amiri, A.; Ghasemi, Y.; Irajie, C. In Silico Design and Evaluation of PRAME+FliCΔD2D3 as a New Breast Cancer Vaccine Candidate. Iran J. Med. Sci. 2021, 46, 52–60.
- Chomez, P.; De Backer, O.; Bertrand, M.; De Plaen, E.; Boon, T.; Lucas, S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001, 61, 5544–5551.
- Wang, Y.; Song, X.; Zheng, Y.; Liu, Z.; Li, Y.; Qian, X.; Pang, X.; Zhang, Y.; Yin, Y. Cancer/testis Antigen MAGEA3 Interacts with STAT1 and Remodels the Tumor Microenvironment. Int. J. Med. Sci. 2018, 15, 1702–1712.
- Dhodapkar, M.V.; Osman, K.; Teruya Feldstein, J.; Filippa, D.; Hedvat, C.V.; Iversen, K.; Kolb, D.; Geller, M.D.; Hassoun, H.; Kewalramani, T.; et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003, 3, 9.
- Xia, L.P.; Xu, M.; Chen, Y.; Shao, W.W. Expression of MAGE-A11 in breast cancer tissues and its effects on the proliferation of breast cancer cells. Mol. Med. Rep. 2013, 7, 254–258.
- Kumar, N.; Sood, D.; Gupta, A.; Jha, N.K.; Jain, P.; Chandra, R. Cytotoxic T-lymphocyte elicited therapeutic vaccine candidate targeting cancer against MAGE-A11 carcinogenic protein. Biosci. Rep. 2020, 40, BSR20202349.
- Blumenthal, R.D.; Leon, E.; Hansen, H.J.; Goldenberg, D.M. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 2007, 7, 2.
- Poola, I.; Shokrani, B.; Bhatnagar, R.; DeWitty, R.L.; Yue, Q.; Bonney, G. Expression of carcinoembryonic antigen cell adhesion molecule 6 oncoprotein in atypical ductal hyperplastic tissues is associated with the development of invasive breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4773–4783.
- Johnson, B.; Mahadevan, D. Emerging Role and Targeting of Carcinoembryonic Antigen-related Cell Adhesion Molecule 6 (CEACAM6) in Human Malignancies. Clin. Cancer Drugs. 2015, 2, 100–111.
