Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) is a key protein responsible for transporting Ca2+ ions from the cytosol into the lumen of the sarco/endoplasmic reticulum (SR/ER), thus maintaining Ca2+ homeostasis within cells. Accumulating evidence suggests that impaired SERCA function is associated with disruption of intracellular Ca2+ homeostasis and induction of ER stress, leading to different chronic pathological conditions. Therefore, appropriate strategies to control Ca2+ homeostasis via modulation of either SERCA pump activity/expression or relevant signaling pathways may represent a useful approach to combat pathological states associated with ER stress. Natural dietary polyphenolic compounds, such as resveratrol, gingerol, ellagic acid, luteolin, or green tea polyphenols, with a number of health-promoting properties, have been described either to increase SERCA activity/expression directly or to affect Ca2+ signaling pathways.
- Ca2+ signaling
- ER stress
- SERCA
- polyphenols
1. Introduction
2. Intracellular Ca2+ Regulation: The Role of SERCA
| SERCA Isoform |
Tissue Distribution | Disease/Complication | SERCA Activity/Expression |
Reference |
|---|---|---|---|---|
| SERCA1a | Adult fast-twitch skeletal muscle |
Brody’s disease | ↓/↓ | [12][36] |
| SERCA1b | Fetal fast-twitch skeletal muscle |
Myotonic dystrophy type 1 | ↓/↑ | [12][37] |
| SERCA2a | Slow twitch skeletal muscle, cardiac muscle, smooth muscle cells | Heart failure Cardiac hypertrophy Diabetic cardiomyopathy Vascular complications Early type 2 diabetes |
↓/↓ -/↓ ↓/↓ ↓/↓ -/↑ |
[12][13][38][39] |
| SERCA2b | All tissues (muscle and non-muscle cells) | Darier’s disease Type 1 and 2 diabetes Cancer Neurodegenerative diseases |
↓/↓ ↓/↓ -/↓ ↓/↓↑ |
[12][36][40][41][42] |
| SERCA2c | Epithelial, mesenchymal, and hematopoietic cells; monocytes |
Cardiomyopathy | -/↑ | [28][36][43] |
| SERCA2d | Skeletal muscle | Myotonic dystrophy type 1 | -/↓ | [37] |
| SERCA3a-f | Non-muscle tissues |
Type 2 diabetes Type 1 diabetes Cardiomyopathy Cancer |
-/↓ -/SERCA3b↑ -/SERCA3f↑ -/↑↓ |
[39][41][43] |
3. Pharmacological Activation of SERCA by Polyphenols
Pharmacological activation of SERCA can reduce ER stress, and may therefore represent a promising therapeutic approach for the treatment of diabetes, metabolic disorders,
cardiovascular diseases (especially heart failure), and neuropathological conditions; alternatively, induction of ER stress by polyphenols may contribute to cancer treatment. Natural polyphenols are able to specifically modulate Ca2+ homeostasis and Ca2+ signaling pathways via SERCA. Polyphenols can affect SERCA by direct binding [71], followed by subsequent changes in its structure and activity. In addition, indirect mechanisms may also lead to alterations in SERCA expression and/or activity. Polyphenol-mediated conformational alterations in either the ATP-binding or Ca2+-binding sites of SERCA are crucial for their protective effects in vivo [72]. To date, most studies on SERCA activation have been conducted regarding the quinoline derivative CDN1163 [73,74]. This small molecular allosteric SERCA activator balances disrupted Ca2+ homeostasis and attenuates diseases associated with ER stress, such as diabetes, metabolic disorders, neurodegenerative problems, or muscular dystrophy [75–77]. Other drug-like SERCA activators, including istaroxime and pyridone derivatives, have been reported to possess stimulatory effects on the cardiac SERCA2a isoform, making them applicable in heart failure treatment [78]. However, there is little information available on the activation of SERCA by natural compounds. Resveratrol, gingerol, ellagic acid, and luteolin belong to the most listed SERCA-targeting compounds in the literature. These compounds exhibit diverse mechanisms of action on the Ca2+ regulatory machinery, from direct interaction with SERCA, through indirect effects via inhibition of SERCA–PLB complex formation, to complex intervention in Ca2+ signaling pathways, thus contributing to various health effects summarizes up-to-date information regarding the effects of polyphenols related to SERCA activation. These compounds exhibit diverse mechanisms of action on the Ca2+ regulatory machinery, from direct interaction with SERCA, through indirect effects via inhibition of–PLB complex formation, to complex intervention in Ca2+ signaling pathways, thus contributing to various health effects. A schematic representation of the major mechanisms of polyphenols’ action with respect to intracellular Ca2+ signaling is depicted in Figure 1.
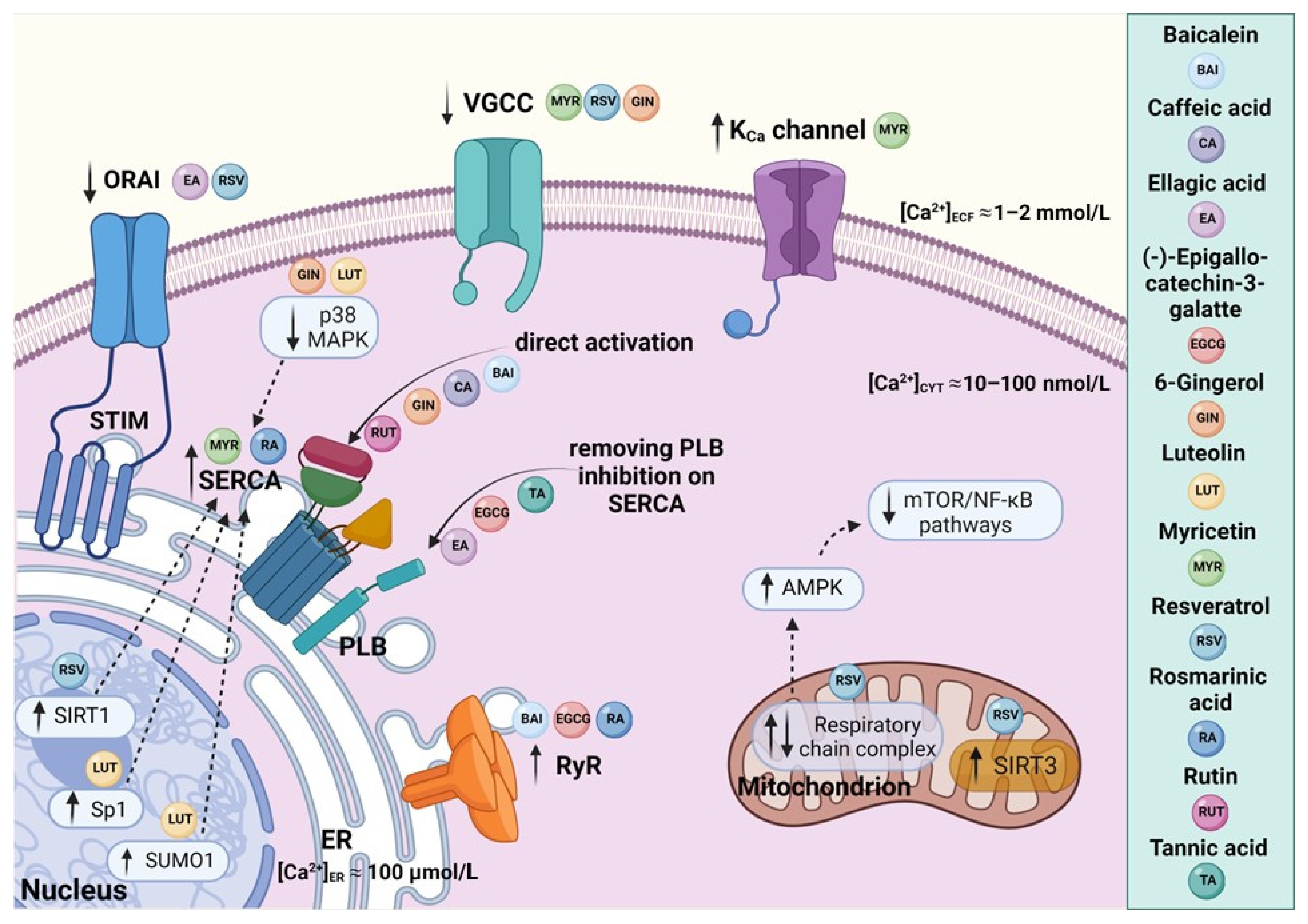 Figure 1. A schematic representation of polyphenol-mediated effects on SERCA and related intracellular Ca2+ signaling pathways: Dietary polyphenols affect Ca2+ dynamics by targeting Ca2+ transporters and channels as well as downstream processes. Baicalein, rutin, caffeic acid, and gingerol seem to stimulate SERCA directly. On the other hand, ellagic acid, (-)-epigallocatechin-3-gallate, and tannins were described as indirect SERCA activators acting by relieving the inhibition of SERCA by PLB. Resveratrol has been shown to interact with several Ca2+-handling proteins, and to modulate Ca2+ homeostasis through intervention in Ca2+ signaling pathways. In particular, the activation of deacetylase SIRT1 has been reported as a central mechanism of resveratrol action responsible for upregulation of SERCA. The release of Ca2+ from the ER via RyRs was shown to be facilitated by baicalein, (-)-epigallocatechin-3-gallate, and rosmarinic acid. Luteolin, myricetin, and rosmarinic acid increase the overexpression of SERCA. The regulatory effects of myricetin, resveratrol, gingerol, and ellagic acid were described in relation to Ca2+-dependent channels, such as VGCCs, ORAI–STIM, and the KCa channel. Figure was created with BioRender.com.
Figure 1. A schematic representation of polyphenol-mediated effects on SERCA and related intracellular Ca2+ signaling pathways: Dietary polyphenols affect Ca2+ dynamics by targeting Ca2+ transporters and channels as well as downstream processes. Baicalein, rutin, caffeic acid, and gingerol seem to stimulate SERCA directly. On the other hand, ellagic acid, (-)-epigallocatechin-3-gallate, and tannins were described as indirect SERCA activators acting by relieving the inhibition of SERCA by PLB. Resveratrol has been shown to interact with several Ca2+-handling proteins, and to modulate Ca2+ homeostasis through intervention in Ca2+ signaling pathways. In particular, the activation of deacetylase SIRT1 has been reported as a central mechanism of resveratrol action responsible for upregulation of SERCA. The release of Ca2+ from the ER via RyRs was shown to be facilitated by baicalein, (-)-epigallocatechin-3-gallate, and rosmarinic acid. Luteolin, myricetin, and rosmarinic acid increase the overexpression of SERCA. The regulatory effects of myricetin, resveratrol, gingerol, and ellagic acid were described in relation to Ca2+-dependent channels, such as VGCCs, ORAI–STIM, and the KCa channel. Figure was created with BioRender.com.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27165095
References
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872.
- Hamilton, S.; Terentyev, D. Proarrhythmic Remodeling of Calcium Homeostasis in Cardiac Disease; Implications for Diabetes and Obesity. Front. Physiol. 2018, 9, 1517.
- Bergantin, L.B. Diabetes and Cancer: Debating the Link through Ca2+/CAMP Signalling. Cancer Lett. 2019, 448, 128–131.
- Eshima, H.; Poole, D.C.; Kano, Y. In Vivo Calcium Regulation in Diabetic Skeletal Muscle. Cell Calcium 2014, 56, 381–389.
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting Calcium Signaling in Cancer Therapy. Acta Pharm. Sin. B 2017, 7, 3.
- Ge, M.; Zhang, J.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of Calcium Homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487–498.
- Jung, H.; Kim, S.Y.; Canbakis Cecen, F.S.; Cho, Y.; Kwon, S.K. Dysfunction of Mitochondrial Ca 2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 599792.
- Missiaen, L.; Robberecht, W.; Van Den Bosch, L.; Callewaert, G.; Parys, J.B.; Wuytack, F.; Raeymaekers, L.; Nilius, B.; Eggermont, J.; De Smedt, H. Abnormal Intracellular Ca2+ Homeostasis and Disease. Cell Calcium 2000, 28, 1–21.
- Feno, S.; Butera, G.; Reane, D.V.; Rizzuto, R.; Raffaello, A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid. Med. Cell. Longev. 2019, 2019, 9324018.
- Stutzmann, G.E.; Mattson, M.P. Endoplasmic Reticulum Ca2+ Handling in Excitable Cells in Health and Disease. Pharmacol. Rev. 2011, 63, 700–727.
- Toyoshima, C. How Ca2+-ATPase Pumps Ions across the Sarcoplasmic Reticulum Membrane. Biochim. Biophys. Acta 2009, 1793, 941–946.
- Periasamy, M.; Kalyanasundaram, A. SERCA Pump Isoforms: Their Role in Calcium Transport and Disease. Muscle Nerve 2007, 35, 430–442.
- Lipskaia, L.; Keuylian, Z.; Blirando, K.; Mougenot, N.; Jacquet, A.; Rouxel, C.; Sghairi, H.; Elaib, Z.; Blaise, R.; Adnot, S.; et al. Expression of Sarco (Endo) Plasmic Reticulum Calcium ATPase (SERCA) System in Normal Mouse Cardiovascular Tissues, Heart Failure and Atherosclerosis. Biochim. Biophys. Acta 2014, 1843, 2705–2718.
- Chemaly, E.R.; Bobe, R.; Adnot, S.; Hajjar, R.J.; Lipskaia, L. Sarco (Endo) Plasmic Reticulum Calcium Atpases (SERCA) Isoforms in the Normal and Diseased Cardiac, Vascular and Skeletal Muscle. J. Cardiovasc. Dis. Diagn. 2013, 1, 1000113.
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263.
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771.
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438.
- Liu, H.; Yang, J.; Li, L.; Shi, W.; Yuan, X.; Wu, L. The Natural Occurring Compounds Targeting Endoplasmic Reticulum Stress. Evid.-Based Complement. Alternat. Med. 2016, 2016, 7831282.
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425.
- Kaneko, M.; Imaizumi, K.; Saito, A.; Kanemoto, S.; Asada, R.; Matsuhisa, K.; Ohtake, Y. ER Stress and Disease: Toward Prevention and Treatment. Biol. Pharm. Bull. 2017, 40, 1337–1343.
- Park, S.W.; Zhou, Y.; Lee, J.; Lee, J.; Ozcan, U. Sarco(Endo)Plasmic Reticulum Ca2+-ATPase 2b Is a Major Regulator of Endoplasmic Reticulum Stress and Glucose Homeostasis in Obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 19320–19325.
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients with Advanced Heart Failure. Circulation 2011, 124, 304–313.
- MacLennan, D.H.; Asahi, M.; Tupling, A.R. The Regulation of SERCA-Type Pumps by Phospholamban and Sarcolipin. Ann. N. Y. Acad. Sci. 2003, 986, 472–480.
- Fajardo, V.A.; Bombardier, E.; Vigna, C.; Devji, T.; Bloemberg, D.; Gamu, D.; Gramolini, A.O.; Quadrilatero, J.; Tupling, A.R. Co-Expression of SERCA Isoforms, Phospholamban and Sarcolipin in Human Skeletal Muscle Fibers. PLoS ONE 2013, 8, e84304.
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T.; et al. Muscle Physiology: A Peptide Encoded by a Transcript Annotated as Long Noncoding RNA Enhances SERCA Activity in Muscle. Science 2016, 351, 271–275.
- Fisher, M.E.; Bovo, E.; Aguayo-Ortiz, R.; Cho, E.E.; Pribadi, M.P.; Dalton, M.P.; Rathod, N.; Lemieux, M.J.; Espinoza-Fonseca, L.M.; Robia, S.L.; et al. Dwarf Open Reading Frame (Dworf) Is a Direct Activator of the Sarcoplasmic Reticulum Calcium Pump Serca. eLife 2021, 10, e65545.
- Lemos, F.O.; Bultynck, G.; Parys, J.B. A Comprehensive Overview of the Complex World of the Endo- and Sarcoplasmic Reticulum Ca2+-Leak Channels. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119020.
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61.
- Vervliet, T.; Parys, J.B.; Bultynck, G. Bcl-2 Proteins and Calcium Signaling: Complexity beneath the Surface. Nat. Publ. Gr. 2016, 35, 5079–5092.
- Rahate, K.; Bhatt, L.K.; Prabhavalkar, K.S. SERCA Stimulation: A Potential Approach in Therapeutics. Chem. Biol. Drug Des. 2020, 95, 5–15.
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788.
- Chen, X.; Cao, R.; Zhong, W. Host Calcium Channels and Pumps in Viral Infections. Cells 2020, 9, 94.
- Cooper, D.; Dimri, M. Biochemistry, Calcium Channels; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium 2014, 56, 340–361.
- Sharma, N.; Arora, S.; Saurav, S.; Motiani, R.K. Pathophysiological significance of calcium signaling at Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs). Curr. Opin. Physiol. 2020, 17, 234–242.
- Hovnanian, A. SERCA pumps and human diseases. Subcell Biochem. 2007, 45, 337–363.
- Nakka, K.; Ghigna, C.; Gabellini, D.; Dilworth, F.J. Diversification of the muscle proteome through alternative splicing. Skelet Muscle 2018, 8, 8.
- Fredersdorf, S.; Thumann, C.; Zimmermann, W.H.; Vetter, R.; Graf, T.; Luchner, A.; Riegger, G.A.; Schunkert, H.; Eschenhagen, T.; Weil, J. Increased myocardial SERCA expression in early type 2 diabetes mellitus is insulin dependent: In vivo and in vitro data. Cardiovasc. Diabetol. 2012, 11, 57.
- Mekahli, D.; Bultynck, G.; Parys, J.B.; de Smedt, H.; Missiaen, L. Endoplasmic-Reticulum Calcium Depletion and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317.
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161.
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Büsselberg, D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019, 20, 3017.
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain. Cell Mol. Neurobiol. 2018, 38, 981–994.
- Dally, S.; Corvazier, E.; Bredoux, R.; Bobe, R.; Enouf, J. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J. Mol. Cell Cardiol. 2010, 48, 633–644.
- Manjarrés, I.M.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The sarco/endoplasmic reticulum Ca(2+) ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium 2010, 47, 412–418.
- Oláh, T.; Fodor, J.; Ruzsnavszky, O.; Vincze, J.; Berbey, C.; Allard, B.; Csernoch, L. Overexpression of transient receptor potential canonical type 1 (TRPC1) alters both store operated calcium entry and depolarization-evoked calcium signals in C2C12 cells. Cell Calcium 2011, 49, 415–425.
- Lee, K.J.; Hyun, C.; Woo, J.S.; Park, C.S.; Kim, D.H.; Lee, E.H. Stromal interaction molecule 1 (STIM1) regulates sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 1a (SERCA1a) in skeletal muscle. Pflug. Arch. Eur. J. Phys. 2014, 466, 987–1001.
- Redondo, P.C.; Salido, G.M.; Pariente, J.A.; Sage, S.O.; Rosado, J.A. SERCA2b and 3 play a regulatory role in store-operated calcium entry in human platelets. Cell Signal 2008, 20, 337–346.
- López, J.J.; Jardín, I.; Bobe, R.; Pariente, J.A.; Enouf, J.; Salido, G.M.; Rosado, J.A. STIM1 regulates acidic Ca2+ store refilling by interaction with SERCA3 in human platelets. Biochem. Pharmacol. 2008, 75, 2157–2164.
