Phosphates are known to be essential for plant growth and development, with phosphorus compounds being involved in various physiological and biochemical reactions. Phosphates are known as one of the most important factors limiting crop yields.
- phosphate solubilization
- soil microbiome
- sustainable agriculture
- biofertilizer
1. Introduction
Phosphorus (P) is one of the most important macronutrients for the growth and development of plants [1][2]. It comprises 0.2 to 0.8% of the dry weight of plants [3] and is found in nucleic acids, enzymes, coenzymes, nucleotides, and phospholipids [4]. Phosphorus is the second most important macronutrient necessary for plants, after nitrogen. The average phosphorus content in soil is about 0.05% (by weight), with only 0.1% of this amount available to be used by plants [5]. Plant roots can absorb phosphorus in the form of orthophosphates H2PO4− or HPO42–, but the concentration of these ions in the soil is in the micromolar range [6]. In soil, most phosphates are present as insoluble iron, aluminum, and calcium phosphates.2. Soil Phosphate-Solubilizing Microorganisms
| Bacteria | Fungi | ||
|---|---|---|---|
| Genera | Ref. | Genera | Ref. |
| Aeromonas | [19] | Achrothcium | [3][20] |
| Agrobacterium | [18] | Alternaria | [3][20] |
| Azotobacter | [21][22] | Arthrobotrys | [3][20] |
| Bacillus | [23] | Aspergillus | [24] |
| Bradyrhizobium | [25] | Cephalosporium | [3][20] |
| Burkholderia | [19][26] | Chaetomium | [3][20] |
| Cyanobacteria | [3] | Cladosporium | [3][20] |
| Enterobacter | [22] | Cunninghamella | [3][20] |
| Erwinia | [27] | Curvularia | [3][20] |
| Kushneria | [5] | Fusarium | [3][20] |
| Micrococcus | [28] | Glomus | [3][20] |
| Paenibacillus | [29] | Helminthosporium | [3][20] |
| Pseudomonas | [30][31] | Micromonospora | [3][20] |
| Rhizobium | [32][33] | Phenomiocenspora | [3][20] |
| Rhodococcus | [25] | Phenomiocenspora | [3][20] |
| Salmonella | [25] | Phenomycylum | [34] |
| Serratia | [35] | Populospora | [3][20] |
| Sinomonas | [25] | Pythium | [3][20] |
| Thiobacillus | [25] | Rhizoctonia | [3][20] |
| Rhizopus | [3][20] | ||
| Saccharomyces | [3][20] | ||
| Schizosaccharomyces | [3][20] | ||
| Schwanniomyces | [3][20] | ||
| Sclerotium | [36] | ||
| Torula | [24] | ||
| Trichoderma | [24] | ||
| Yarrowia | [3][20] | ||
3. Biochemical Properties of the Soil Related to the Bioavailability of Phosphates
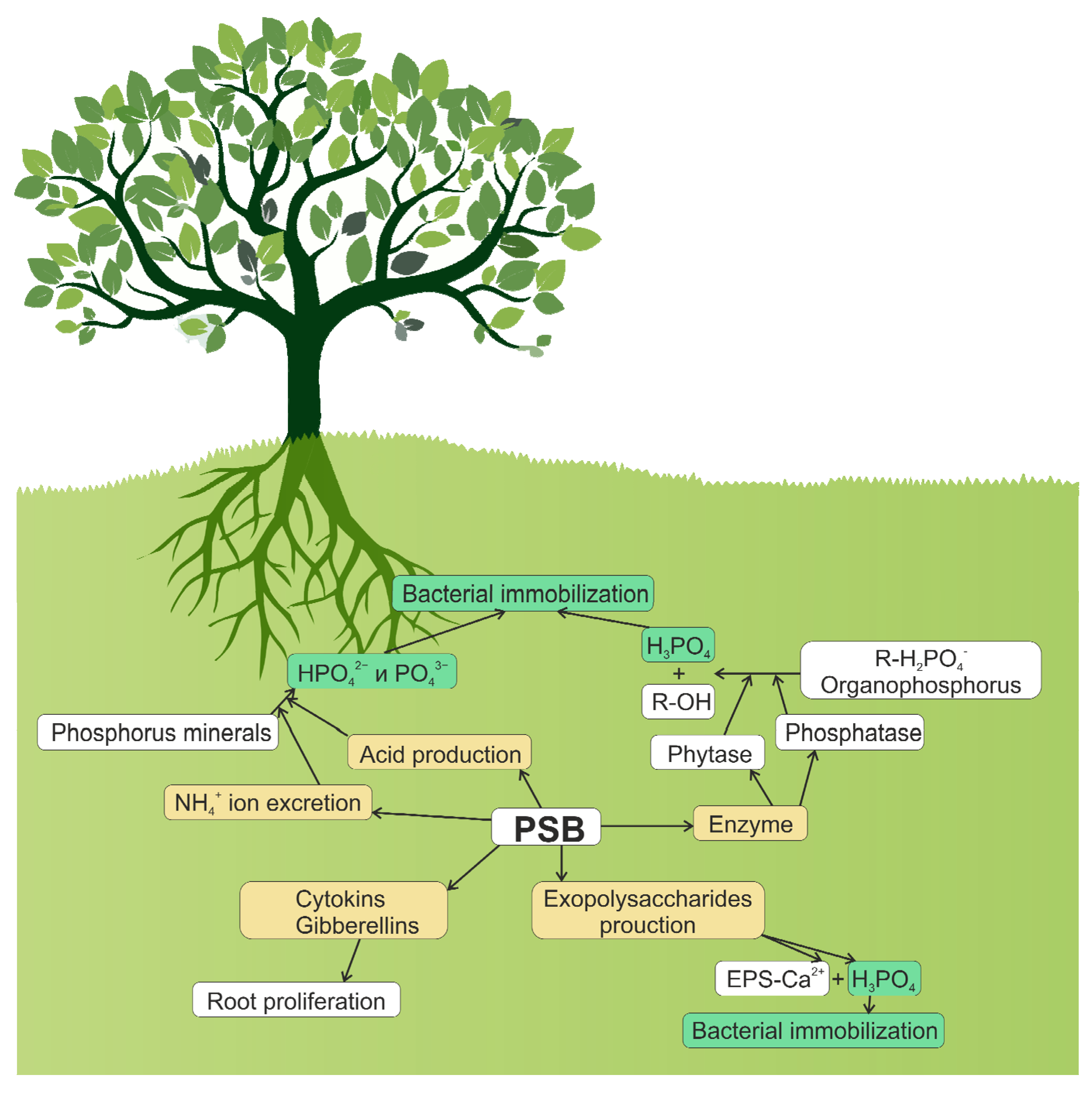
4. Mechanisms of Inorganic Phosphate Solubilization
| Phosphate-Solubilizing Microorganism | Ecological Niche | Predominantly Produced Acids | Ref. |
|---|---|---|---|
| Escherichia freundii | Soil | Lactic | [62] |
| Aspergillus niger, Penicillium sp. | Soil | Citric, glycolic, succinic, gluconic, oxalic, lactic | [62] |
| Bacillus megaterium, Pseudomonas sp., Bacillus subtilus | Soil rizoshpere | Lactic, malic | [63] |
| Arthrobacter sp., Bascillus sp., Bacillus firmus B-7650 | Wheat and cowpea rhizosphere | Lactic, citric | [64] |
| Aspergillus sp., Penicillium sp., Chaetomium nigricolor | Lateritic soil | Oxalic, succinic, citric, 2-ketogluconic | [65] |
| A. japonicus, A. foetidus | Indian rock phosphate | Oxalic, citric, gluconic, succinic, tartaric | [66] |
| P. radicum | Wheat rhizosphere | Gluconic | [67] |
| Enterobacter agglomerans | Wheat rhizosphere | Oxalic, citric | [68] |
| Bacillus amyloliquefaciens, B. licheniformis, B. atrophaeus, Penibacillus macerans, Vibrio proteolyticus, Xanthobacter agilis, Enterobacter aerogenes, E. taylorae, E. asburiae, Kluyvera cryocrescens, Pseudomonas aeromonassens, Chrysler | Mangrove | Lactic, itaconic, isovaleric, isobutyric, acetic | [69] |
| Penicillium rugulosum | Venezuelan phosphate rocks | Citric, gluconic acid | [70] |
| Enterobacter intermedius | Grass rhizosphere | 2-ketogluconic | [71] |
| Aspergillus flavus, A. niger, Penicillium canescens | Wheat grains | Oxalic, citric, gluconic, succinic | [72] |
| Pseudomonas fluorescens | Oil palms rhizosphere | Citric, malic, tartaric, gluconic | [73] |
| Aspergillus niger | Tropical and subtropical soils | Gluconic, oxalic | [74] |
| P. trivialis | Rhizosphere of Hippophae rhamnoides (cold Howl and Spiti deserts, Trans-Himalayas) | Lactic, formic | [75] |
| B. pumilus var.2; B. subtilis var.2; Actinomadura oligospora; Citrobacter sp. | Giant cardon cactus (P. pringlei) | Gluconic, propionic, isovaleric, heptonic, caproic, isocaproic, formic, valeric, succinic, oxalic, oxaloacetic, malonic | [76] |
| B. pumilus CHOO8A; B. fusiformis | Opuntia cholla | gluconic, oxalic, 2-ketogluconic, lactic, succinic aid, formic, citric, malic | [76] |
| Bacillus sp. SENDO 6 и | P. pringlei | Gluconic, propionic, isovaleric, formic, succinic, lactic | [77] |
| Pseudomonas putida M5TSA, Enterobacter sakazakii M2PFe и Bacillus megaterium M1PCa | Mammillaria fraileana cactus | Gluconic, propionic, acetic, formic, succinic, lactic, oxalic | [78] |
5. Mineralization of Organic Phosphate-Containing Compounds
The content of organic phosphorus in the soil can reach 30–50% of the total amount, with soil organic phosphorus being found primarily in the form of inositol phosphate (soil phytate). Other organic phosphorus compounds were reported, including: phosphomonoesters, phosphodiesters, phospholipids, nucleic acids, and phosphotriesters [18]. Additionally, large quantities of xenobiotics (pesticides, detergents, antibiotics, and flame retardants) that are regularly released into the environment are also known to contain organic P. Most of these organic compounds are of high molecular weight and resistant to chemical hydrolysis. For plant uptake, these compounds must be converted into soluble ionic phosphate (Pi, HPO42−, H2PO4−) or low molecular weight organic phosphates [87].
Several groups of enzymes secreted by phosphate-solubilizing microorganisms are involved in the process of phosphate mineralization. The enzymes of the first group dephosphorylate phosphor-ester or phosphoanhydride organic compounds. These are non-specific acid phosphatases (NSAP). The most studied NSAP enzymes are phosphomonoesterases, also called phosphatases [88]. These enzymes can be acidic or alkaline phosphomonoesterases [89]. The pH of the soils possessing phosphatase activity was indicated to be from acidic to neutral, indicating that acid phosphatases play a major role in this process [90].
Phytase is another phosphate-solubilizing enzyme involved in the mineralization of organic P. This enzyme is responsible for releasing phosphorus from organic compounds in the soil (plant seeds and pollen) that are stored in the form of phytate (inositol polyphosphate). Phytase releases phosphates in a form that is available to plants. While plants cannot obtain phosphorus directly from the phytate, the presence of phosphate-solubilizing microbes in the rhizosphere can compensate for the inability of plants to obtain phosphates directly from phytate [91]. Phytates are synthesized by plants and constitute a significant amount (from 60 to 80%) of organic phosphorus in the soil. However, the ester bonds in phytic acid are quite stable and their natural degradation is practically impossible. Microbial mineralization of phytate by phytase plays an essential role in the process of phosphorus recycling. Phytate can be completely hydrolyzed with the formation of one inositol and six molecules of inorganic phosphate, or partially with the formation of lower isomers of inositol polyphosphate and inorganic phosphates [92].
Among the four types of phytases identified, β-propeller phytase (BPP, EC 3.1.3.8 or EC 3.1.3.26) differs from the other three phytases (histidine acid phosphatase, cysteine phytase, and purple acid phosphatase) in that it has a neutral ∼pH 7.0 rather than an acidic pH optimum. It has been indicated that BPP is the main class of phytate degrading enzymes in nature. A typical BPP has a six-lobed propeller fold with two phosphate-binding sites (a cleavage site and an affinity site) and six calcium-binding sites, three of which are high-affinity binding sites responsible for enzyme stability and three of which are low-affinity sites, regulating the catalytic activity of the enzyme [93][94].
Thus far, only a small number of BPP have been isolated and studied, including Shewanella oneidensis MR-1 PhyS, Bacillus subtilis PhyC, Bacillus sp. DS11 Phy, B. subtilis 168 168PhyA, Bacillus licheniformis PhyL, Pedobacterobsis 5 MJ11 PhyP, and Janthinobacterium sp. TN115 PhyA115, all of which are mesophilic or thermophilic. Phosphate solubilization by acid phosphatases was reported for Pseudomonas sp., Burkholderia cepacia, Enterobacter aerogenes, E. cloacae, Citrobacter freundi, Proteus mirabalis, and Serratia marcenscens. Moreover, solubilization of organic phosphate by phytase activity was observed in Bacillus subtilis, Pseudomonas putida, and P. mendocina, and phosphatase activity was discovered in Klebsiella aerogenes and P. fluorescens. However, although P. sonchi SBR5 possesses some of these enzymes associated with phosphate solubilization, the activation of the corresponding genes was not observed when differential gene expression was analyzed under the phosphate solubilization conditions.
The production of phosphatases by the soil microbiome was proved to be tightly controlled by the availability of inorganic phosphorus and nitrogen. The addition of nitrogen increases the phosphatase activity, while the supply of inorganic phosphorus suppresses the production and activity of phosphatases due to the negative feedback mechanism [95].
Phytase-producing fungi are, A. fumigatus, A. niger, A. parasiticus, A. rugulosus, A. terreus, Penicillium rubrum, P. simplicissimum, Pseudeurotium zonatum, Trichoderma harzianum, and Trichoderma viride. Soil Bacillus and Streptomyces spp. are able to mineralize complex organic phosphates by producing extracellular enzymes such as phosphoesterases, phosphodiesterases, phytases, and phospholipases [96].
6. Perspectives of Use
To enhance the use of PSBs as effective and important components in sustainable soil management systems, more data in needed on the molecular mechanisms they use to increase the bioavailability of phosphates. Consumers pay attention to the health, quality, and nutritional value of agricultural products. Thus, applying phosphate-solubilizing microorganisms as biofertilizers is one option to increase food production without posing a health risk, while saving natural sources of phosphate fertilizers and developing sustainable agriculture. It is important for researchers to continue studying phosphate-solubilizing microorganisms and translate this knowledge into a form that can be easily used by farmers [97].
The papers published in the recent years indicate that the efficiency of using phosphates in agriculture could be improved by inoculation with phosphate-solubilizing bacteria, which increase the availability of phosphate without disturbing the biochemical composition of the soil. These potential biofertilizers are universal since they can be used for various crops and generally are not specific for plants. Conversely, the development of personalized, plant-specific consortia of phosphate-solubilizers is likely to increase productivity further. Inoculation of phosphate-solubilizing microorganisms into the soil appears to be an effective way to convert insoluble phosphate compounds into bioavailable forms, resulting in better plant growth, yield, and quality. In this research, researchers demonstrate that the bacteria of genera Bacillus, Pseudomonas, Rhizobium, Aspergillus, and Penicillium genera are regarded as the most effective phosphate-solubilizers for increasing the phosphate bioavailability in soil. PSBs cause immediate plant growth by providing phosphates in an easily absorbable form. Additionally, phosphate-solubilizing microorganisms support plant growth by increasing the efficiency of nitrogen fixation. Two main mechanisms increasing free phosphate in the soil can be distinguished: the mobilization of inorganic and organic phosphates. In general, the mobilization of inorganic phosphate is performed primarily due to the release of organic acids by bacteria, and enzymes released into the extracellular environment perform the mineralization of organic phosphates.
While most farmers rely on inorganic sources of phosphates with which to avoid nutrient deficiencies, significant amounts of these fertilizers are lost from the soil through various mechanisms and are unavailable for plant uptake. In legume cropping systems, phosphate deficiency can also lead to nitrogen deficiency and reduced crop yields. Since the PSB-based biofertilizers have indicated promising effects on plant growth and yield, researchers assume that the phosphate-solubilizing microorganisms may be potential substitutes for inorganic phosphate fertilizers as a method to meet plant requirements and consequently increase the yields in sustainable agriculture. Their application is an environmentally and economically sound approach.
Solubilization of inorganic phosphate in the soil is found to increase its bioavailability for the plant, thus using PSBs in the soil is to promote sustainable agriculture, improve soil fertility, and increase crop yields. Use of PSB as microbial inoculants is considered as the new frontier for increasing plant productivity. This technology can contribute to low-cost farming systems and a cleaner environment.
This entry is adapted from the peer-reviewed paper 10.3390/plants11162119
References
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ramakrishnan, M.; Duraipandiyan, V.; Abdulla, A.-D.N.; Ignacimuthu, S. Utilization of molecular markers for improving the phosphorus efficiency in crop plants. Plant Breed. 2018, 137, 10–26.
- Santana, E.B.; Marques, E.L.S.; Dias, J.C.T. Effects of phosphate-solubilizing bacteria, native microorganisms, and rock dust on Jatropha curcas L. growth. Genet. Mol. Res. 2016, 15, 1–18.
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587.
- Nesme, T.; Metson, G.S.; Bennett, E.M. Global phosphorus flows through agricultural trade. Glob. Environ. Chang. 2018, 50, 133–141.
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneriasp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based Complementary Altern. Med. 2011, 2011, 615032.
- Ai, P.; Sun, S.; Zhao, J.; Fan, X.; Xin, W.; Guo, Q.; Yu, L.; Shen, Q.; Wu, P.; Miller, A.J.; et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809.
- Azziz, G.; Bajsa, N.; Haghjou, T.; Taulé, C.; Valverde, Á.; Igual, J.M.; Arias, A. Abundance, diversity and prospecting of culturable phosphate solubilizing bacteria on soils under crop–pasture rotations in a no-tillage regime in Uruguay. Appl. Soil Ecol. 2012, 61, 320–326.
- Schnug, E.; Haneklaus, S.H. The Enigma of Fertilizer Phosphorus Utilization. In Phosphorus in Agriculture: 100% Zero; Springer: Dordrecht, The Netherlands, 2016; pp. 7–26.
- Chaney, R.L. Food Safety Issues for Mineral and Organic Fertilizers. Adv. Agron. 2012, 117, 51–116.
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350.
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43.
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54.
- Wani, P.; Khan, M.; Zaidi, A. Co-inoculation of nitrogen-fixing and phosphate-solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron. Hung. 2007, 55, 315–323.
- Wang, H.-Y.; Liu, S.; Zhai, L.-M.; Zhang, J.-Z.; Ren, T.-Z.; Fan, B.-Q.; Liu, H.-B. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167.
- Alori, E.; Fawole, O.; Afolayan, A. Characterization of Arbuscular Mycorrhizal Spores Isolated from Southern Guinea Savanna of Nigeria. J. Agric. Sci. 2012, 4, 13.
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119.
- Browne, P.; Rice, O.; Miller, S.H.; Burke, J.; Dowling, D.N.; Morrissey, J.P.; O’Gara, F. Superior inorganic phosphate solubilization is linked to phylogeny within the Pseudomonas fluorescens complex. Appl. Soil Ecol. 2009, 43, 131–138.
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339.
- Jha, A.; Sharma, D.; Saxena, J. Effect of single and dual phosphate-solubilizing bacterial strain inoculations on overall growth of mung bean plants. Arch. Agron. Soil Sci. 2012, 58, 967–981.
- Srinivasan, R.; Yandigeri, M.S.; Kashyap, S.; Alagawadi, A.R. Effect of salt on survival and P-solubilization potential of phosphate solubilizing microorganisms from salt affected soils. Saudi J. Biol. Sci. 2012, 19, 427–434.
- Kumar, S.; Bauddh, K.; Barman, S.C.; Singh, R.P. Amendments of microbial biofertilizers and organic substances reduces requirement of urea and DAP with enhanced nutrient availability and productivity of wheat (Triticum aestivum L.). Ecol. Eng. 2014, 71, 432–437.
- Jiang, Z.; Zhang, X.; Wang, Z.; Cao, B.; Deng, S.; Bi, M.; Zhang, Y. Enhanced biodegradation of atrazine by Arthrobacter sp. DNS10 during co-culture with a phosphorus solubilizing bacteria: Enterobacter sp. P1. Ecotoxicol. Environ. Saf. 2019, 172, 159–166.
- Jahan, M.; Mahallati, M.N.; Amiri, M.B.; Ehyayi, H.R. Radiation absorption and use efficiency of sesame as affected by biofertilizers inoculation in a low input cropping system. Ind. Crop. Prod. 2013, 43, 606–611.
- Zhao, L.; Zhang, Y.-Q. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597.
- Postma, J.; Nijhuis, E.H.; Someus, E. Selection of phosphorus solubilizing bacteria with biocontrol potential for growth in phosphorus rich animal bone charcoal. Appl. Soil Ecol. 2010, 46, 464–469.
- Mamta; Rahi, P.; Pathania, V.; Gulati, A.; Singh, B.; Bhanwra, R.K.; Tewari, R. Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside-A contents of Stevia rebaudiana Bertoni. Appl. Soil Ecol. 2010, 46, 222–229.
- Chakraborty, U.; Chakraborty, B.N.; Basnet, M.; Chakraborty, A.P. Evaluation of Ochrobactrum anthropic TRS-2 and its talc based formulation for enhancement of growth of tea plants and management of brown root rot disease. J. Appl. Microbiol. 2009, 107, 625–634.
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010, 48, 987–992.
- Bidondo, L.F.; Silvani, V.; Colombo, R.; Pérgola, M.; Bompadre, J.; Godeas, A. Pre-symbiotic and symbiotic interactions between Glomus intraradices and two Paenibacillus species isolated from AM propagules. In Vitro and in vivo assays with soybean (AG043RG) as plant host. Soil Biol. Biochem. 2011, 43, 1866–1872.
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745.
- Paul, D.; Sinha, S.N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136.
- Tajini, F.; Trabelsi, M.; Drevon, J.-J. Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 2012, 19, 157–163.
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C.; Tola, Y.H. Co-inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front. Plant Sci. 2017, 8, 141.
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313.
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178.
- Thampi, A.; Bhai, R.S. Rhizosphere actinobacteria for combating Phytophthora capsici and Sclerotium rolfsii, the major soil borne pathogens of black pepper (Piper nigrum L.). Biol. Control 2017, 109, 1–13.
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256.
- Arai, Y.; Sparks, D. Phosphate Reaction Dynamics in Soils and Soil Components: A Multiscale Approach. Adv. Agron. 2007, 94, 135–179.
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174.
- Mnkeni, P.N.S.; MacKenzie, A.F. Retention of Ortho- and Polyphosphates in Some Quebec Soils as Affected by Added Organic Residues and Calcium Carbonate. Can. J. Soil Sci. 1985, 65, 575–585.
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners—The roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 2019, 434, 7–45.
- Ehlers, K.; Bakken, L.R.; Frostegård, Å.; Frossard, E.; Bünemann, E.K. Phosphorus limitation in a Ferralsol: Impact on microbial activity and cell internal P pools. Soil Biol. Biochem. 2010, 42, 558–566.
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996.
- Kulaev, I.S.; Vagabov, V.M. Polyphosphate Metabolism in Micro-Organisms. Adv. Microb. Physiol. 1983, 24, 83–171.
- Hirota, R.; Kuroda, A.; Kato, J.; Ohtake, H. Bacterial phosphate metabolism and its application to phosphorus recovery and industrial bioprocesses. J. Biosci. Bioeng. 2010, 109, 423–432.
- Kuroda, A.; Murphy, H.; Cashel, M.; Kornberg, A. Guanosine Tetra- and Pentaphosphate Promote Accumulation of Inorganic Polyphosphate in Escherichia coli. J. Biol. Chem. 1997, 272, 21240–21243.
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210.
- Karl, D.M. Microbially Mediated Transformations of Phosphorus in the Sea: New Views of an Old Cycle. Annu. Rev. Mar. Sci. 2014, 6, 279–337.
- Seeling, B.; Zasoski, R.J. Microbial effects in maintaining organic and inorganic solution phosphorus concentrations in a grassland topsoil. Plant Soil 1993, 148, 277–284.
- Khan, K.S.; Joergensen, R.G. Changes in microbial biomass and P fractions in biogenic household waste compost amended with inorganic P fertilizers. Bioresour. Technol. 2009, 100, 303–309.
- Sugito, T.; Yoshida, K.; Takebe, M.; Shinano, T.; Toyota, K. Soil microbial biomass phosphorus as an indicator of phosphorus availability in a Gleyic Andosol. Soil Sci. Plant Nutr. 2010, 56, 390–398.
- Spohn, M.; Widdig, M. Turnover of carbon and phosphorus in the microbial biomass depending on phosphorus availability. Soil Biol. Biochem. 2017, 113, 53–59.
- Malik, M.A. Phosphorus fractions, microbial biomass and enzyme activities in some alkaline calcareous subtropical soils. Afr. J. Biotechnol. 2012, 11, 4773–4781.
- Chen, G.C.; He, Z.L. Microbial biomass phosphorus turnover in variable-charge soils in China. Commun. Soil Sci. Plant Anal. 2002, 33, 2101–2117.
- Dinh, M.-V.; Guhr, A.; Spohn, M.; Matzner, E. Release of phosphorus from soil bacterial and fungal biomass following drying/rewetting. Soil Biol. Biochem. 2017, 110, 1–7.
- Patel, D.K.; Archana, G.; Kumar, G.N. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Curr. Microbiol. 2008, 56, 168–174.
- Vijayakumar, A.; Rajasekharan, R. Distinct Roles of Alpha/Beta Hydrolase Domain Containing Proteins. Biochem. Mol. Biol. J. 2016, 2, 1–3.
- Son, H.-J.; Park, G.-T.; Cha, M.-S.; Heo, M.-S. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210.
- Zhao, K.; Penttinen, P.; Zhang, X.; Ao, X.; Liu, M.; Yu, X.; Chen, Q. Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014, 169, 76–82.
- Asea, P.; Kucey, R.; Stewart, J. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol. Biochem. 1988, 20, 459–464.
- Illmer, P.; Schinner, F. Solubilization of inorganic calcium phosphates—Solubilization mechanisms. Soil Biol. Biochem. 1995, 27, 257–263.
- Kouas, S.; Labidi, N.; Debez, A.; Abdelly, C. Effect of P on nodule formation and N fixation in bean. Agron. Sustain. Dev. 2005, 25, 389–393.
- Taha, S.M.; Mahmoud, S.A.Z.; El-Damaty, A.H.; El-Hafez, A.M. Activity of phosphate-dissolving bacteria in Egyptian soils. Plant Soil 1969, 31, 149–160.
- Bajpai, P.D.; Rao, W.V.B.S. Phosphate solubilising bacteria. Soil Sci. Plant Nutr. 1971, 17, 46–53.
- Banik, S.; Dey, B.K. Phosphate-Solubilizing Potentiality of the Microorganisms Capable of Utilizing Aluminium Phosphate as a Sole Phosphate Source. Zentralbl. Mikrobiol. 1983, 138, 17–23.
- Singal, R.; Gupta, R.; Saxena, R.K. Rock phosphate solubilization under alkaline conditions by Aspergillus japonicus and A. foetidus. Folia Microbiol. 1994, 39, 33–36.
- Whitelaw, M.A.; Harden, T.J.; Helyar, K.R. Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biol. Biochem. 1999, 31, 655–665.
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352.
- Vazquez, P.; Holguin, G.; Puente, M.E.; Lopez-Cortes, A.; Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 2000, 30, 460–468.
- Reyes, I.; Baziramakenga, R.; Bernier, L.; Antoun, H. Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol. Biochem. 2001, 33, 1741–1747.
- Kim, K.Y.; Hwangbo, H.; Park, R.D.; Kim, Y.W.; Rim, Y.S.; Park, K.H.; Kim, T.H.; Suh, J.S. 2-Ketogluconic Acid Production and Phosphate Solubilization by Enterobacter intermedium. Curr. Microbiol. 2003, 47, 87–92.
- Rashid, M.; Khalil, S.; Ayub, N.; Alam, S.; Latif, F. Organic Acids Production and Phosphate Solubilization by Phosphate Solubilizing Microorganisms (PSM) Under in vitro Conditions. Pak. J. Biol. Sci. 2004, 7, 187–196.
- Fankem, H.; Nwaga, D.; Deube, A.; Dieng, L.; Merbach, W.; Etoa, F. Occurrence and functioning of phosphate solubilizing microorganisms from oil palm tree (Elaeis guineensis) rhizosphere in Cameroon. Afr. J. Biotechnol. 2006, 5, 2450–2460.
- Chuang, C.-C.; Kuo, Y.-L.; Chao, C.-C.; Chao, W.-L. Solubilization of inorganic phosphates and plant growth promotion by Aspergillus niger. Biol. Fertil. Soils 2007, 43, 575–584.
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174.
- Puente, M.E.; Li, C.Y.; Bashan, Y. Microbial Populations and Activities in the Rhizoplane of Rock-Weathering Desert Plants. II. Growth Promotion of Cactus Seedlings. Plant Biol. 2004, 6, 643–650.
- Puente, M.E.; Li, C.Y.; Bashan, Y. Rock-degrading endophytic bacteria in cacti. Environ. Exp. Bot. 2009, 66, 389–401.
- Lopez, B.R.; Bashan, Y.; Bacilio, M. Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the southern Sonoran Desert. Arch. Microbiol. 2011, 193, 527–541.
- Oubrie, A.; Rozeboom, H.J.; Kalk, K.H.; Olsthoorn, A.J.; Duine, J.A.; Dijkstra, B.W. Structure and mechanism of soluble quinoprotein glucose dehydrogenase. EMBO J. 1999, 18, 5187–5194.
- Lessie, T.G.; Phibbs, P.V. Alternative Pathways of Carbohydrate Utilization in Pseudomonads. Annu. Rev. Microbiol. 1984, 38, 359–388.
- Thomas, L.; Hodgson, D.A.; Wentzel, A.; Nieselt, K.; Ellingsen, T.E.; Moore, J.; Morrissey, E.R.; Legaie, R.; The STREAM Consortium; Wohlleben, W.; et al. Metabolic Switches and Adaptations Deduced from the Proteomes of Streptomyces coelicolor Wild Type and phoP Mutant Grown in Batch Culture. Mol. Cell. Proteom. 2012, 11, M111.013797.
- Buch, A.; Archana, G.; Kumar, G.N. Metabolic channeling of glucose towards gluconate in phosphate-solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res. Microbiol. 2008, 159, 635–642.
- Zeng, Q.; Wu, X.; Wen, X. Effects of Soluble Phosphate on Phosphate-Solubilizing Characteristics and Expression of gcd Gene in Pseudomonas frederiksbergensis JW-SD2. Curr. Microbiol. 2016, 72, 198–206.
- Ben Farhat, M.; Fourati, A.; Chouayekh, H. Coexpression of the Pyrroloquinoline Quinone and Glucose Dehydrogenase Genes from Serratia marcescens CTM 50650 Conferred High Mineral Phosphate-Solubilizing Ability to Escherichia coli. Appl. Biochem. Biotechnol. 2013, 170, 1738–1750.
- Liu, S.T.; Lee, L.Y.; Tai, C.Y.; Hung, C.-H.; Chang, Y.S.; Wolfram, J.H.; Rogers, R.; Goldstein, A.H. Cloning of an Erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in Escherichia coli HB101: Nucleotide sequence and probable involvement in biosynthesis of the coenzyme pyrroloquinoline quinone. J. Bacteriol. 1992, 174, 5814–5819.
- Zeng, Q.; Wu, X.; Wang, J.; Ding, X. Phosphate Solubilization and Gene Expression of Phosphate-Solubilizing Bacterium Burkholderia multivorans WS-FJ9 under Different Levels of Soluble Phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855.
- Peix, A.; Mateos, P.F.; Rodriguez-Barrueco, C.; Martínez-Molina, E.; Velazquez, E. Growth promotion of common bean (Phaseolus vulgaris L.) by a strain of Burkholderia cepacia under growth chamber conditions. Soil Biol. Biochem. 2001, 33, 1927–1935.
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243.
- Jorquera, M.A.; Crowley, D.E.; Marschner, P.; Greiner, R.; Fernández, M.T.; Romero, D.; Menezes-Blackburn, D.; De La Luz Mora, M. Identification of β-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus spp. from the rhizosphere of pasture plants on volcanic soils. FEMS Microbiol. Ecol. 2011, 75, 163–172.
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339.
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996.
- Rao, D.E.C.S.; Rao, K.V.; Reddy, T.P.; Reddy, V.D. Molecular characterization, physicochemical properties, known and potential applications of phytases: An overview. Crit. Rev. Biotechnol. 2009, 29, 182–198.
- Kim, O.-H.; Kim, Y.-O.; Shim, J.-H.; Jung, Y.-S.; Jung, W.-J.; Choi, W.-C.; Lee, H.; Lee, S.-J.; Kim, K.-K.; Auh, J.-H.; et al. β-Propeller Phytase Hydrolyzes Insoluble Ca2+-Phytate Salts and Completely Abrogates the Ability of Phytate to Chelate Metal Ions. Biochemistry 2010, 49, 10216–10227.
- Fu, S.; Sun, J.; Qian, L. Effect of Ca2+ on beta-propeller phytases. Protein Pept. Lett. 2008, 15, 39–42.
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704.
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971.
