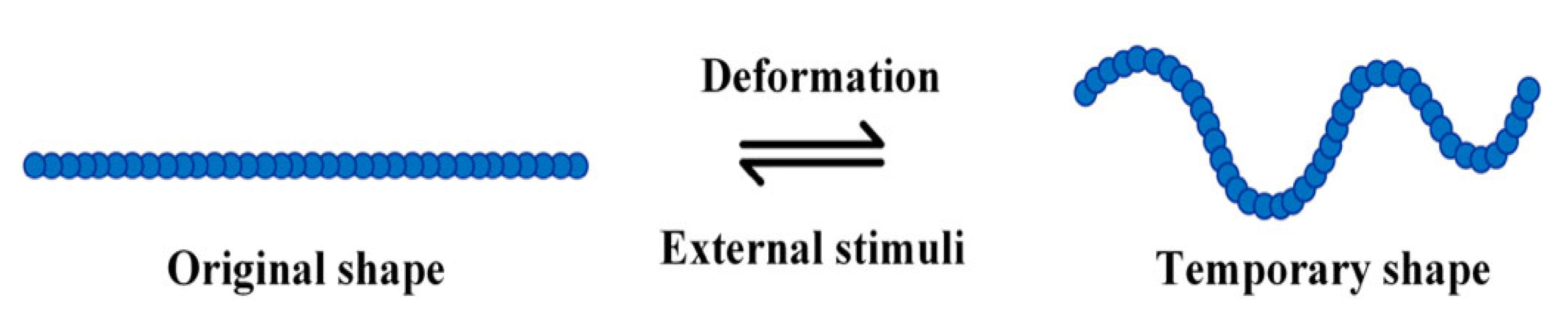
Shape memory elastomers have revolutionised the world since their introduction in the 20th century. The ability to tailor chemical structures to produce a family of materials in wide-ranging forms with versatile properties has propelled them to be ubiquitous.
- shape memory
- smart polymers
- elastomers
- biotechnology
1. Introduction
|
Polymer |
Elastomer |
|---|---|
|
Range from highly crosslinked to no crosslinks |
Slightly crosslinked |
|
Range from being flexible to rigid |
Flexible and elastic |
|
Wide range of glass transition temperatures |
Typically low (sub-zero) glass transition temperature |
|
Elastomer |
Trigger |
Potential Applications |
References |
|---|---|---|---|
|
Tetrathiafulvalene polymer networks |
Thermal, Light |
Thermoactuating smart sunshade and sensors |
[43] |
|
Polyurethane |
Thermal, pH, UV irradiation |
Pressure bandages, Bone tissue engineering, Smart textiles, Self-healing materials, Shielding, Heat-shrinkable films, Stent materials, etc. |
|
|
Polybutadiene |
Thermal |
Self-healing materials, Automobiles, Electronics, Medical equipment, Sports materials, and Shoes. |
[48] |
|
Polystyrene |
Thermal |
Sealants, Coatings, Adhesives, and Automotive parts. |
[49] |
|
Poly(ε–caprolactone) |
Thermal, Water, Light |
Water-responsive sensors, Actuators, Medical devices, Self-tightening sutures, Smart stents, and Artificial scaffolds. |
|
|
Poly(ether–b–amide) |
Moisture |
Biomedical and industrial applications |
[54] |
|
Polyamide elastomers |
Thermal |
Self-healing materials, Intelligent electronic devices, Bionic materials |
[55] |
|
Products |
Growth Rate |
Value (USD) |
|---|---|---|
|
Sports shoes [60] |
31% |
2320 million |
|
Bedding [61] |
17% |
115 million |
|
Thermoplastic elastomers [62] |
5.8% |
5.1 billion |
2. Thermodynamic Aspects Governing SMEs
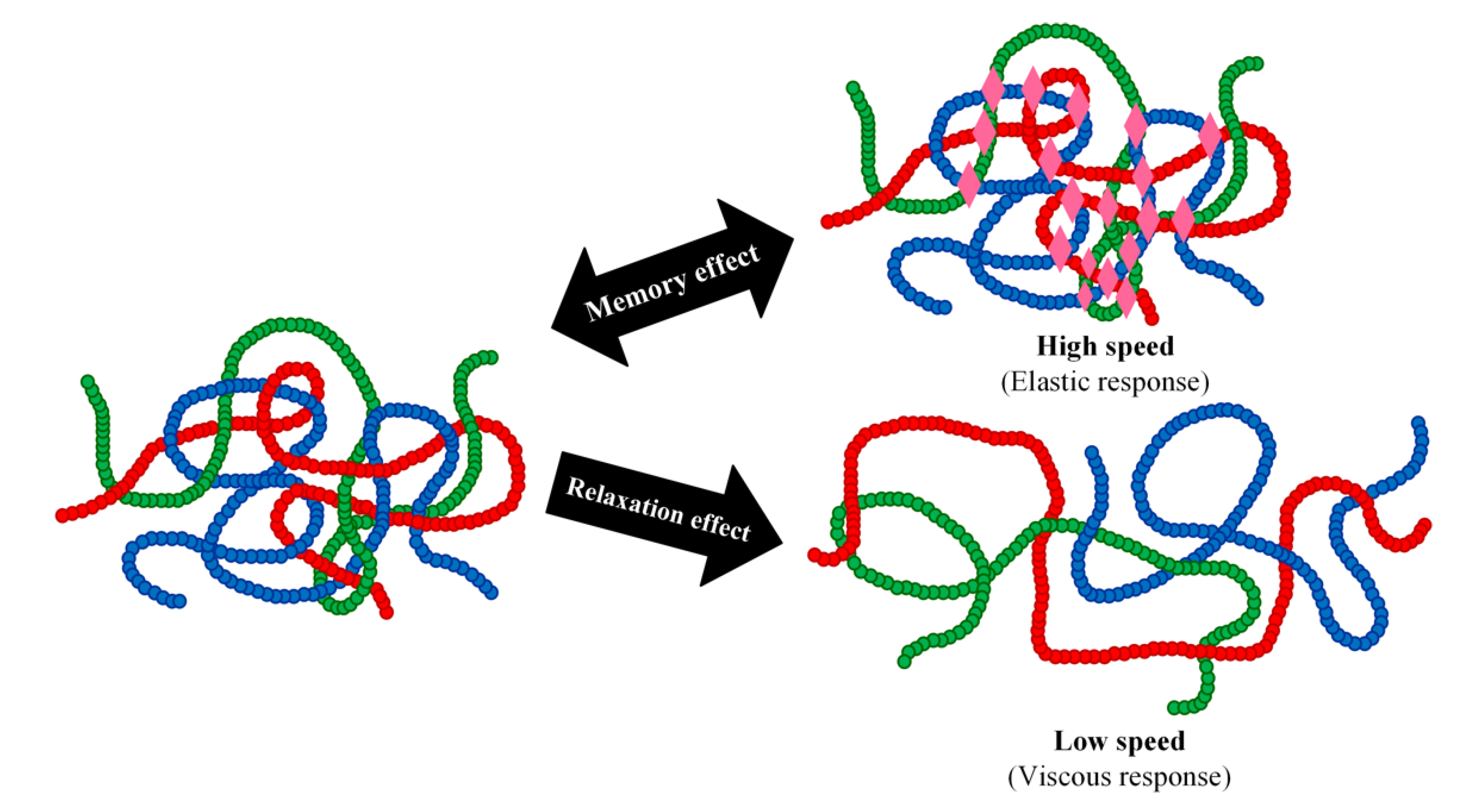
This entry is adapted from the peer-reviewed paper 10.3390/polym14163276
References
- Kamila, S. Introduction, Classification and applications of smart materials: An overview. Am. J. Appl. Sci. 2013, 10, 876–880.
- Khoo, Z.; Teoh, J.; Liu, Y.; Chua, C.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122.
- Kim, H.C.; Mun, S.; Ko, H.U.; Zhai, L.; Kafy, A.; Kim, J. Renewable smart materials. Smart Mater. Struct. 2016, 25, 073001.
- Yang, H.; Ma, L. Multi–stable mechanical metamaterials by elastic buckling instability. J. Mater. Sci. 2019, 54, 3509–3526.
- Brighenti, R.; Li, Y.; Vernerey, F.J. Smart polymers for advanced applications: A mechanical perspective review. Front. Mater. 2020, 7, 196.
- Sobczyk, M.; Wiesenhütter, S.; Noennig, J.R.; Wallmersperger, T. Smart materials in architecture for actuator and sensor applications: A review. J. Intell. Mater. Syst. Struct. 2021, 33, 379–399.
- Panwar, S.; Panjagari, N.R.; Singh, A.K.; Deshwal, G.K.; Badola, R.; Minz, P.S.; Goksen, G.; Rusu, A.; Trif, M. Electrospun smart oxygen indicating tag for modified atmosphere packaging applications: Fabrication, characterization and storage stability. Polymers 2022, 14, 2108.
- Reneker, D.H.; Mattice, W.L.; Quirk, R.P.; Kim, S.J. Macromolecular smart materials and structures. Smart Mater. Struct. 1992, 1, 84–90.
- Cavicchi, K.A. Shape memory polymers from blends of elastomers and small molecule additives. Macromol. Symp. 2015, 358, 194–201.
- Drossel, W.G.; Kunze, H.; Bucht, A.; Weisheit, L.; Pagel, K. Smart3—Smart materials for smart applications. Procedia CIRP 2015, 36, 211–216.
- Wang, J.; Xue, Z.; Li, G.; Wang, Y.; Fu, X.; Zhong, W.H.; Yang, X. A UV–curable epoxy with “soft” segments for 3D–printable shape–memory materials. J. Mater. Sci. 2018, 53, 12650–12661.
- Meurer, J.; Bätz, T.; Hniopek, J.; Jäger, M.; Zechel, S.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Synthesis and characterization of metallopolymer networks featuring triple shape–memory ability based on different reversible metal complexes. Polymers 2022, 14, 1833.
- Khadem, E.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Das, O.; Berto, F. Cutting–edge progress in stimuli–responsive bioadhesives: From synthesis to clinical applications. Polymers 2022, 14, 1709.
- González-Jiménez, A.; Bernal-Ortega, P.; Salamanca, F.M.; Valentin, J.L. Shape–memory composites based on ionic elastomers. Polymers 2022, 14, 1230.
- Gunes, I.; Cao, F.; Jana, S. Evaluation of nanoparticulate fillers for shape memory polyurethane nanocomposites. Polymer 2008, 49, 2223–2234.
- Huang, W.M.; Ding, Z.; Wang, C.C.; Wei, J.; Zhao, Y.; Purnawali, H. Shape memory materials. Mater. Today 2010, 13, 54–61.
- Kuriyagawa, M.; Kawamura, T.; Hayashi, S.; Nitta, K.-h. Effects of addition of hindered phenol compounds to a segmented polyurethane with shape memory on mechanical yielding. J. Mater. Sci. 2011, 46, 1264–1271.
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape–memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135.
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763.
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33.
- Lin, T.; Li, S.; Ke, J.; Zheng, Y.; Yu, Y. Unique shape memory elastomer associated with reversible sacrificial hydrogen bonds: Tough and flexible when below its Tg. Adv. Eng. Mater. 2018, 20, 1800051.
- Chen, H.-M.; Wang, L.; Zhou, S.-B. Recent progress in shape memory polymers for biomedical applications. Chin. J. Polym. Sci. 2018, 36, 905–917.
- Hasan, S.M.; Touchet, T.; Jayadeep, A.; Maitland, D.J. Controlling morphology and physio–chemical properties of stimulus–responsive polyurethane foams by altering chemical blowing agent content. Polymers 2022, 14, 2288.
- Deng, X.; Chen, G.; Liao, Y.; Lu, X.; Hu, S.; Gan, T.; Handschuh-Wang, S.; Zhang, X. Self–healable and recyclable dual-shape memory liquid metal—Elastomer composites. Polymers 2022, 14, 2259.
- Chen, G.; Chen, D. Heterogeneous solid-state plasticity of a multi-functional metallo-supramolecular shape-memory polymer towards arbitrary shape programming. Polymers 2022, 14, 1598.
- Liu, C.; Qin, H.; Mather, P.T. Mather, Review of progress in shape–memory polymers. J. Mater. Chem. 2007, 17, 1543–1558.
- Rousseau, I.A. Challenges of shape memory polymers: A review of the progress toward overcoming SMP’s limitations. Polym. Eng. Sci. 2008, 48, 2075–2089.
- Oliver, K.; Seddon, A.; Trask, R.S. Morphing in nature and beyond: A review of natural and synthetic shape-changing materials and mechanisms. J. Mater. Sci. 2016, 51, 10663–10689.
- Karger-Kocsis, J.; Kéki, S. Review of progress in shape memory epoxies and their composites. Polymers 2018, 10, 34.
- Wang, L.; Luo, B.; Wu, D.; Liu, Y.; Li, L.; Liu, H. Fabrication and characterization of thermal-responsive biomimetic small-scale shape memory wood composites with high tensile strength, high anisotropy. Polymers 2019, 11, 1892.
- Luo, X.; Mather, P.T. Preparation and characterization of shape memory elastomeric composites. Macromolecules 2009, 42, 7251–7253.
- Li, J.; Rodgers, W.R.; Xie, T. Semi–crystalline two–way shape memory elastomer. Polymer 2011, 52, 5320–5325.
- Belmonte, A.; Guzmán, D.; Fernández-Francos, X.; de la Flor, S. Effect of the network structure and programming temperature on the shape–memory response of thiol-epoxy “click” systems. Polymers 2015, 7, 2146–2164.
- Song, X.; Chi, H.; Li, Z.; Li, T.; Wang, F. Star–shaped crosslinker for multifunctional shape memory polyurethane. Polymers 2020, 12, 740.
- Cardarelli, F. Materials Handbook: A Concise Desktop Reference, 2nd ed.; Springer: London, UK, 2008; p. 1339.
- McKeen, L.W. The Effect of Sterilization on Plastics and Elastomers, 3rd ed.; PDL Handbook Series; William Andrew: Norwich, NY, USA, 2012.
- Suethao, S.; Shah, D.U.; Smitthipong, W. Recent progress in processing functionally graded polymer foams. Materials 2020, 13, 4060.
- Suethao, S.; Ponloa, W.; Phongphanphanee, S.; Wong-Ekkabut, J.; Smitthipong, W. Current challenges in thermodynamic aspects of rubber foam. Sci. Rep. 2021, 11, 6097.
- Suethao, S.; Phongphanphanee, S.; Wong-ekkabut, J.; Smitthipong, W. The relationship between the morphology and elasticity of natural rubber foam based on the concentration of the chemical blowing agent. Polymers 2021, 13, 1091.
- Sun, L.; Huang, W.M.; Wang, T.X.; Chen, H.M.; Renata, C.; He, L.W.; Lv, P.; Wang, C.C. An overview of elastic polymeric shape memory materials for comfort fitting. Mater. Des. 2017, 136, 238–248.
- Wee, J.S.-H.; Chai, A.B.; Ho, J.-H. Fabrication of shape memory natural rubber using palmitic acid. J. King Saud Univ. Sci. 2017, 29, 494–501.
- Lovšin, M.; Brandl, D.; Glavan, G.; Belyaeva, I.A.; Cmok, L.; Čoga, L.; Kalin, M.; Shamonin, M.; Drevenšek-Olenik, I. Reconfigurable surface micropatterns based on the magnetic field–induced shape memory effect in magnetoactive elastomers. Polymers 2021, 13, 4422.
- Rim, M.; Kang, D.-G.; Ko, H.; Wi, Y.; Kim, W.; Kim, S.; Hyeong, J.; Kim, N.; Jeong, K.-U. Shape–morphing thermoactuators: Tetrathiafulvalene–based polymer networks with an effective phonon conduction pathway. Chem. Mater. 2022, 34, 718–727.
- Chen, H.; Li, Y.; Liu, Y.; Gong, T.; Wang, L.; Zhou, S. Highly pH–sensitive polyurethane exhibiting shape memory and drug release. Polym. Chem. 2014, 5, 5168–5174.
- Thakur, S.; Hu, J. Polyurethane: A shape memory polymer (SMP). In Aspects of Polyurethanes; Yılmaz, F., Ed.; InTech: Rijeka, Croatia, 2017; pp. 53–71.
- Gupta, A.; Maharjan, A.; Kim, B.S. Shape memory polyurethane and its composites for various applications. Appl. Sci. 2019, 9, 4694.
- Hu, J.; Feng, Z.; Xu, X.; Gao, W.; Ning, N.; Yu, B.; Zhang, L.; Tian, M. UV reconfigurable shape memory polyurethane with a high recovery ratio under large deformation. Ind. Eng. Chem. Res. 2021, 60, 2144–2153.
- Wu, W.; Zhou, Y.; Li, J.; Wan, C. Shape memory and self–healing behavior of styrene–butadiene–styrene/ethylene–methacrylic acid copolymer (SBS/EMAA) elastomers containing ionic interactions. J. Appl. Polym. Sci. 2020, 137, 48666.
- Pantoja, M.; Jian, P.-Z.; Cakmak, M.; Cavicchi, K.A. Shape memory properties of polystyrene–block–poly(ethylene–co–butylene)–block–polystyrene (SEBS) ABA triblock copolymer thermoplastic elastomers. ACS Appl. Polym. Mater. 2019, 1, 414–424.
- Gu, X.; Mather, P.T. Water–triggered shape memory of multiblock thermoplastic polyurethanes (TPUs). RSC Adv. 2013, 3, 15783–15791.
- Niu, G. Water triggered shape memory materials. Sci. Insights 2013, 3, 49–50.
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self–healing and shape memory polycaprolactone–based polymers. Eur. Polym. J. 2018, 101, 169–176.
- Zhang, Y.; Zhou, S.; Chong, K.C.; Wang, S.; Liu, B. Near–infrared light–induced shape memory, self–healable and anti–bacterial elastomers prepared by incorporation of a diketopyrrolopyrrole–based conjugated polymer. Mater. Chem. Front. 2019, 3, 836–841.
- Shibasaki, Y.; Mori, T.; Fujimori, A.; Jikei, M.; Sawada, H.; Oishi, Y. Poly(amide–ether) thermoplastic elastomers based on monodisperse aromatic amide hard segments as shape–memory and moisture–responsive materials. Macromolecules 2018, 51, 9430–9441.
- Chen, Z.; Li, Y.; Yao, C. Biomass shape memory elastomers with rapid self–healing properties and high recyclability. Biomacromolecules 2021, 22, 2768–2776.
- Prasopdee, T.; Smitthipong, W. Effect of fillers on the recovery of rubber foam: From theory to applications. Polymers 2020, 12, 2745.
- Lin, J.-Y.; Lin, M.-C.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H.; Chen, Y.-S. Novel composite planks made of shape memory polyurethane foaming material with two–step foaming process. Polymers 2022, 14, 275.
- Wang, T.X.; Renata, C.; Chen, H.M.; Huang, W.M. Elastic shape memory hybrids programmable at around body–temperature for comfort fitting. Polymers 2017, 9, 674.
- Rupérez, M.J.; Monserrat, C.; Alemany, S.; Juan, M.C.; Alcañíz, M. Contact model, fit process and, foot animation for the virtual simulator of the footwear comfort. Comput. Aided Des. 2010, 42, 425–431.
- International Trade Centre. List of Exporters for the Selected Product in 2021. Product: 640319 Sports Footwear, with Outer Soles of Rubber, Plastics, Leather or Composition Leather and Uppers of Leather (Excluding Ski–Boots, Cross–Country Ski Footwear, Snowboard Boots and Skating Boots with Ice or Roller Skates Attached). 2021. Available online: https://www.trademap.org/Country_SelPro-uct.aspx?nvpm=1%7c%7c%7c%7c%7c640319%7c%7c%7c6%7c1%7c1%7c2%7c1%7c1%7c2%7c1%7c1%7c1 (accessed on 25 June 2022).
- International Trade Centre. List of Exporters for the Selected Product in 2021. Product: 940490 “Articles of Bedding and Similar Furnishing, Fitted with Springs or Stuffed or Internally Filled with Any Material or of Cellular Rubber or Plastics (Excl. Mattress Supports, Mattresses, Sleeping Bags, Pneumatic or Water Mattresses, Blankets, Covers, Quilts, Bedspreads, Eiderdowns and Duvets “Comforters”). 2021. Available online: https://www.trademap.org/Country_SelProduct.aspx?nvpm=1%7c%7c%7c%7c%7c940490%7c%7c%7c6%7c1%7c1%7c1%7c1%7c1%7c2%7c1%7c1%7c1 (accessed on 25 June 2022).
- MarketsandMarkets Research Private Ltd. Arkema S.A (France) and BASF SE (Germany) Are Leading Players in the Construction Elastomers Market 2021. Available online: https://www.marketsandmarkets.com/ResearchInsight/construction–elastomers–market.asp (accessed on 25 June 2022).
- Behl, M.; Lendlein, A. Shape–memory polymers. Mater. Today 2007, 10, 20–28.
- Dolynchuk, O.; Kolesov, I.; Radusch, H.-J. Thermodynamic description and modeling of two–way shape–memory effect in crosslinked semicrystalline polymers. Polym. Adv. Technol. 2014, 25, 1307–1314.
- Oikonomou, P.; Sanopoulou, M.; Papadokostaki, K.G. Blends of poly(vinyl alcohol) and poly(vinyl pyrrolidone): Interrelation between the degree of hydration and thermal and mechanical properties. Ind. Eng. Chem. Res. 2021, 60, 14203–14212.
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J.-M. Shape–memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294.
- Pakornpadungsit, P.; Smitthipong, W.; Chworos, A. Self–assembly nucleic acid–based biopolymers: Learn from the nature. J. Polym. Res. 2018, 25, 45.
