Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Craniopharyngiomas (CPs) currently represent one of the most challenging diseases to deal with in the group of skull base tumors. Due to their location near, within, or surrounding the pituitary gland and stalk, CPs can be revealed by pituitary tumor syndrome and/or symptoms of hormonal deficiencies.
- craniopharyngioma
- adamantinomatous
- papillary
1. Introduction
Craniopharyngiomas (CPs) are rare brain tumors resulting from malformations of embryonic remnants along the original pathway of the craniopharyngeal duct [1]. Overall, CPs comprise 1.2 to 4.6% of all intracranial tumors, with an incidence of histologically confirmed cases of 0.16/100,000 persons per year in the USA [2]. In spite of belonging to the group of benign epithelial tumors, according to the World Health Organization [3], CPs are highly problematic in the clinical field because of the hormonal and hypothalamic disorders that they cause. In children, a recent observational study found a high prevalence of early endocrine disorders after brain tumors, including in children suffering from CPs [4]. Similar outcomes are seen in the adult population, with complications of visual, pituitary, and/or hypothalamic function, all of these regions being exposed to surgically induced damage that is associated with tumor resection. Moreover, cases of malignant CPs exist, even though their definition/presentation is not currently particularly well described [5,6,7]. CPs are divided into two distinct subtypes, adamantinomatous CP (ACP) and papillary CP (PCP), differing both in histological features and genetic alterations [8] (Figure 1). ACP is the most prevalent subtype seen in children and adults, displaying a bimodal age distribution, with peaks between the ages of 5–15 years and 45–60 years. Conversely, PCPs are almost exclusively encountered in adults. At the genetic level, somatic mutations in CTNNB1 (encoding β-catenin) are found in roughly 90% of ACPs, leading to the activation of the WNT pathway, while PCPs frequently harbor somatic BRAFV600E mutations that result in the activation of the mitogen-activated protein kinase (MAPK) signaling pathway [9].
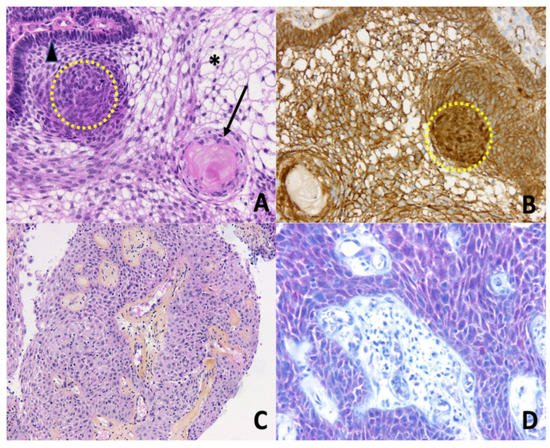
Figure 1. Histopathology of craniopharyngiomas. (A) Adamantinomatous craniopharyngioma. Epithelial nests with peripheral palisading columnar epithelium (arrowhead), nodular whorls (yellow dotted circle), stellate reticulum (asterisk), and aggregates of ‘wet’ keratin (black arrow) (Hematoxylin Phloxine Saffron (HPS) staining, ×200); (B) Adamantinomatous craniopharyngioma. Nucleocytoplasmic translocation and accumulation of β-catenin are detected by immunohistochemistry, especially in the nodular whorls (brown nuclear immunopositivity, yellow dotted circle, ×200); (C) Papillary craniopharyngioma. Papillae are composed of fibrovascular cores covered by a well-differentiated non-keratinizing squamous epithelium (HPS, ×100); (D) Papillary craniopharyngioma. Detection of BRAF V600E mutation by immunohistochemistry is positive (red cytoplasmic immunopositivity in squamous neoplastic cells, ×200).
Symptoms at diagnosis in adults differ from those seen in children, with frequent visual alterations as presenting features of the patient because of the tumor mass effect. Headaches can be moderate; however, their chronic presentation makes them unusual and worrying for the patient. When present, endocrine deficiencies are responsible for individual impairments such as sexual dysfunction or polydipsia/polyuria. In parallel, patients may complain of disabilities affecting their social and professional lives, such as a decline in cognitive function with a significant impact on their job performance [10,11,12]. Albeit rare before surgery, a hypothalamic syndrome may be suspected preoperatively, when disruptions in body temperature regulation, growth, and water balance or eating behavior disorders are present. Even today, the prognosis in adult patients with CPs constitutes an important issue, as a 3-fold overall mortality rate and an up to 19-fold higher cerebrovascular mortality rate have been reported as compared to the general population [13,14,15]. Indeed, even when disease control is achieved, related disabilities frequently require the intervention of multiple health care professionals with, sometimes, an incapacity to return to normal professional activity. For CPs, one current challenging discussion concerns the treatment strategy which, for years, has relied almost exclusively on surgery and radiotherapy. Recent progress that has been made in deciphering the molecular background of CPs now paves the way for new therapeutic approaches.
2. Management of Craniopharyngiomas: A Multimodal Approach
Except for small localized craniopharyngiomas (CP) that can benefit from close monitoring, the classical management of CP most often involves a multimodal approach that may combine one or more surgical operations with various modalities of radiotherapy (including intra-cystic treatments). The following paragraphs briefly detail the main therapeutic modalities for CP, keeping in mind that these approaches should always be discussed in an expert center by a multidisciplinary team [16].
2.1. Surgery
Surgery can be proposed in several settings: as an emergency for chiasmatic decompression or reduction of intracranial pressure; as a preventive measure to avoid chiasmatic compression or intracranial hypertension in the case of recurrence; or to reduce the tumor volume which may then be subjected to radiotherapy [17]. The question of the degree of extension of the surgery has been controversial: some have advocated radical surgery, which could either lead to the removal of the pituitary stalk and/or damage to the hypothalamic structures, while others have proposed less radical surgery, with a higher risk of recurrence but less morbidity for the surrounding structures (especially the hypothalamus). The aim of treatment is to avoid or control recurrence, at the cost of a low risk of per- and postoperative morbidity. In particular, the risk of hypothalamic dysfunction induced by surgery, while sometimes already present at diagnosis, is one of the main criteria to consider [18]. The risk of complications induced by the degree of surgical resection can be assessed by preoperative MRI, which allows visualization of the degree of adhesion or compression of hypothalamic structures. Several classifications of hypothalamic morbidity correlated with post-operative abnormalities and the status of the third ventricle floor have been reported [19]. Of note, any surgery for tumor recurrence is associated with lower surgical efficacy than the original surgery, and an increased risk of complications, including mortality, which increasingly leads to a preference for second-stage radiotherapy instead of surgical revision. For pure cystic CPs, a cystic aperture in the third ventricle (sometimes with a sub-cutaneous reservoir) followed by a wait-and-see strategy may also represent a safe option [20].
2.2. Irradiation
Different modalities of radiation therapy have been evaluated in patients with CP. They include conventional external radiotherapy, proton beam therapy, stereotactic radiotherapy, and radiosurgery. Usually, these modalities are used in combination with partial surgery, or more rarely alone [21,22]. When combined with surgery, radiation techniques can be employed in two different settings: immediately after an incomplete surgery (partial surgery decided by the surgeon), or after surgery, during the follow-up of the patient, when a new tumor remnant appears, or if a previously known tumor remnant becomes progressive (radiotherapy as a second step). In that setting, the notion of slow progressiveness is important, since radiotherapy does not have an immediate effect on tumor volume, with a decrease in tumor volume generally starting from 6 to 12 months after the procedure and continuing over time. Historically, the most common modality of radiotherapy used in patients with CPs consisted of the delivery of a fractionated dose of photons (i.e., conventional external radiotherapy). With this approach, volume control of the tumor residue is high, estimated at nearly 80–90% by some studies. Another type of conformational therapy can be used with protons instead of photons (Proton therapy). It has the theoretical advantage of low proton scattering on the surrounding structures, thus reducing the risk of complications [23]. However, efficacy data for this approach remains rather limited to date.
Techniques of radiation therapy using a stereotactic frame can be either used with fractionation (stereotactic radiotherapy) or with the delivery of a single fraction (also called radiosurgery). Interestingly, their precision is precious to spare adjacent structures. Radio-surgery modalities include Gamma Knife or cyber-knife. Data on pituitary tumors suggest a better tolerance but a lower efficacy of these more precise approaches compared to conventionally fractionated radiotherapy [24].
2.3. Intra-Cystic Treatments
Cysts are frequently found in the case of CPs and can therefore develop in the anatomical spaces nearby the pituitary sella. Amongst them, the formation of cysts occurring in the third ventricle is one of the most frequent localization of cystic development: it can lead to obstructive hydrocephalus either as the calling-in point at the initial diagnosis and/or in case of recurrence. It may require the use of an intra-cystic catheter or even an Ommaya reservoir to allow repeated evacuation of the cystic fluid. Regarding intra-cystic treatments, the data available in the literature is currently scarce. This approach only applies to craniopharyngiomas with exclusively, or predominantly, cystic recurrence. Intra-cystic treatment with gamma-interferon may be useful in about ¼ of cases, without a clear predictive factor of efficacy. A recent study demonstrated an absence of recurrence at 14 months of follow-up in 14 of 56 children treated with this approach [25]. Other molecules (bleomycin) or radioisotopes (90Yttrium and 32Phosphorus) have been proposed: in the absence of robust published data, the risk-benefit ratio does not seem generally to be in favor of this type of treatment [26]. However, a discussion may be held in a multidisciplinary consultation board to determine an individualized approach.
2.4. Side Effects and Quality of Life
2.4.1. Side Effects of Treatments
Side effects consist mainly of the occurrence of new hormonal deficiencies, in addition to those already present at diagnosis, the latter being mostly central hypogonadism and GH deficiency. Hypothalamic damage may also lead to obesity. Hormonal deficiencies justify lifelong hormone replacement therapy (pituitary deficiencies including AVP deficiency). The question of growth hormone (GH) replacement therapy and the possible risk of recurrence must be discussed on a case-by-case basis, as emphasized by recent recommendations concerning intracranial tumors [27]. Although in vitro data suggests a proliferative effect of GH in cell culture [28], clinical data are actually very reassuring and GH substitution appears to be beneficial for patients with GH deficiency [29,30,31]. The management of hypothalamic obesity is complex and has for a long time relied solely on strict dietary management. Growth hormones can also improve body composition in this setting. The potential benefits of medicinal (GLP-1 analogues, oxytocin) or surgical approaches (bariatric surgery) are currently being studied to evaluate their efficacy and their possible side effects [32,33,34].
Radiotherapy, especially if performed in the pediatric age group, is an effective treatment, but may expose the patient to later risk of radiation-induced tumors, cognitive disorders, or vascular thrombosis [35]. The indication for and modality of radiotherapy should therefore be determined by a multidisciplinary tumor board. Most retrospective studies evaluating the risks of radiotherapy have been conducted using older modalities in order to obtain data with a sufficiently prolonged follow-up. The most modern modalities (radiosurgery, stereotactic radiotherapy, and proton beam therapy) represent promising tools that deserve to be further evaluated in the specific context of craniopharyngiomas. Progress in the delivery of proton therapy could lead to a preference for this modality in the future because it could potentially reduce the risk of side effects; however, this remains to be demonstrated.
2.4.2. Quality of Life in Patients with Craniopharyngiomas
Patients with CP have an altered quality of life and often have cognitive or attentional problems [36,37]. However, there are specific data in the literature on the long-term quality of life of patients with CP, compared by therapeutic approach in terms of the different modalities of radiotherapy, or the approach of repeated surgery versus surgery combined with radiotherapy. It is likely that the degree of aggressiveness of its management (the number of different procedures, extension of surgery and associated post-operative morbidities) is correlated with the long-term quality of life, as was suggested in the short term (three-year follow-up) study from the KRANIOPARYNGEOM registry [38]. Psychological care is essential for all patients from the beginning of the treatment, and at each disease recurrence.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14153831
This entry is offline, you can click here to edit this entry!
