Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Colorectal cancer, being mostly colorectal carcinomas (CRC), represents the third most diagnosed cancer and the second cause of cancer-related death. Molecular classification serves as indicator of targeted therapy
- colorectal cancer
- molecular classification
[1]1. Consensus Molecular Subtype Classification
Differences in tumor behavior and response to therapy in same-stage CRC cases have increased the need for gene-expression studies and the creation of a molecular classification that would facilitate targeted therapy [2,4,36,37,38,39]. In this regard, four consensus molecular subtypes (CMS 1-4) were introduced in 2005, based on multiple molecular characteristics and the presence or absence of epithelial-mesenchymal transition (EMT) [39].
Tumors belonging to the CMS1 subtype are hypermutated, with BRAF mutant status, microsatellite instability (MSI-H), and an important immune reaction. CMS1 group is also known as MSI immune. Carcinogenesis seems to be driven via JAK-STAT and PD-1 signaling pathways [39,40]. Although the pathways are similar for MSI and MSS cases belonging to this group, MSS carcinomas’ behavior and answer to therapy are also influenced by CD8+ cytotoxic T cell infiltration amount [40].
CMS2 (canonical) and CMS3 (metabolic) represent epithelial subtypes. CMS2 is chromosomally unstable, with activation of WNT and MYC signaling pathways. CMS3 shows metabolic deregulations and KRAS mutations and comprises MSI-H and one-third of cases that are microsatellite stable (MSS). The CMS3 MSS-carcinomas are architecturally such as MSI tumors [36,37,38,39,40,41].
CRCs with stromal invasion, angiogenesis, and transforming growth factor β (TGF-ß) activation are included in the CMS4 subtype, which is also known as the mesenchymal subtype [36,37,38,39,40,41]. Hypermethylation of the miR-200 family’s promoter was associated with stimulation of the EMT process in this mesenchymal subtype, frequently diagnosed in advanced stages and associated with worse survival parameters and activation of vascular endothelial growth factor (VEGF) and TGF-ß [38,42]. A risk stratification formula based on the expression of six immune genes, recently described by Zhang et al. might become useful in the clinical management of CMS4-type CRC [43].
In one of the recent studies, a refined classification of the CMS2 and CMS3 (epithelial cases) was proposed based on intrinsic epithelial subtype (I), microsatellite instability status (M) and fibrosis (F). It was called “iCMS” or “IMF” classification but implementation in daily practice is not easy to be performed [41].
Studies confirm response and outcome differences between tumors included in the four CMSs, larger cohorts being required for any valid official changes [44,45,46,47]. CMS1, MSI immune subtype, mostly identified in CRC of the right colon, seems to respond well to immunotherapy and to show better prognosis when bevacizumab, a VEGF inhibitor, is associated with the classical treatment scheme [37,44]. Although immunotherapy shows promising results for MSI-H cases from the CMS1 group, the CMS1-MSS carcinomas do not respond to immune checkpoint inhibitors [40].
Heterogenous research results indicate better overall survival (OS) for CRCs CMS2 and CMS3 when bevacizumab or cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, is associated with classical therapy [37,44,45]. The latter also showed significant benefits when used for BRAF/RAS wild type, left-sided metastatic CRCs [42]. Adding cetuximab or irinotecan to CMS4-CRC chemotherapy appears to be more beneficial than adding bevacizumab or oxaliplatin-based therapy [47,48]. As KRAS mutations can be identified in CMS4 carcinomas, resistance to cetuximab should be considered [40].
Besides aiding the molecular classification process, microsatellite status by itself shows important diagnostic and therapeutic implications. It represents the presence of repeated sequences encompassing 1-6 nucleotides, causing mutations of the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2), mutations that can be inherited (Lynch syndrome) or developed sporadically [36,37]. Screening for mutations of these genes or loss of IHC expression of their corresponding proteins enables the selection of MMR-deficient/MSI-high tumors, which are known to respond to fluoropyrimidine-based therapy and immunotherapy (pembrolizumab and nivolumab being recently approved by the Food and Drug Administration) [36,37,38,41].
2. Immunohistochemical-Based Molecular Classification
Multiple studies attempted to molecularly classify CRC using the expression of IHC antibodies for legit reasons such as cost-efficiency and availability in most pathology departments [48,49,50,51,52]. Most research studies used the following panel of antibodies: cytokeratin, CDX2 for epithelial-like tumors, FRMD6, ZEB1, HTR2B for mesenchymal-like tumors, and determination of microsatellite status [50,52,53].
These stains were not enough for the distinction between CMS2 and CMS3, which are mainly driven via the Wnt pathway [40]. Li X. et al. recently added β-catenin to the above-mentioned panel, considering positive nuclear expression an indicator of CMS2, because CTNNB1, the gene encoding β-catenin, appeared to be upregulated in this subtype [49].
Here will focus on classifying CRC based on IHC reactions and used markers of EMT such as E-cadherin, β- catenin, vimentin, and maspin, evaluated in both tumor center and invasion front/tumor buds (Figure 2) [51,54,55]. The researchers contoured three subtypes: epithelial (diffuse membrane expression of E-cadherin and β-catenin associated with negative vimentin), mesenchymal (loss of E-cadherin expression, positive vimentin and nuclear staining of β-catenin and maspin) and one with mixed epithelial-mesenchymal features called hybrid (epithelial-like pattern in the tumor center and mesenchymal characteristics in the invasion front), all of them exemplified in Figure 2 [51,56].
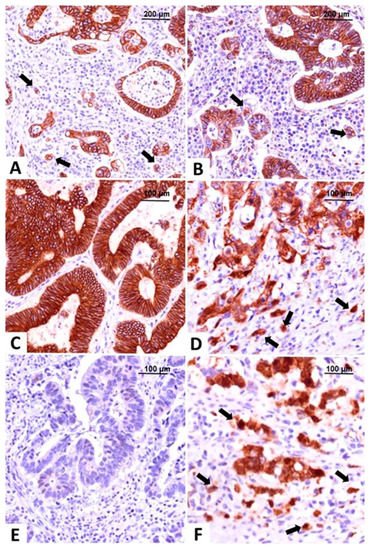
Figure 2. Molecular classification of colorectal carcinomas based on the immunohistochemical expression of E-cadherin and β-catenin. The epithelial subtype is easily recognized by diffuse membrane staining for E-cadherin (A) and β-catenin (B), in the core and tumor buds (indicated with arrows). The intermediate, hybrid subtype, presents epithelial-type expression in the tumor center, with membrane expression of E-cadherin (C) and β-catenin (D), and buds with mesenchymal immunophenotype showing nuclear β-catenin, indicated with arrows (D). The mesenchymal subtype does not stain for E-cadherin (E) and β-catenin (F) is predominantly nuclear, in both tumor center and buds, indicated with arrows (F). Pictures from the personal collection of authors—referenced data published in 2020–2021 [51,56].
Tumor budding, defined as the single tumor cells or groups of no more than four tumor cells identified in the invasion front, represents an extensively studied parameter with a Hematoxylin-Eosin +/− cytokeratin slide-based evaluation protocol published in 2017, represents an independent prognostic marker not yet included in AJCC staging manual, but its importance and suggestion for addition in the pathological report are mentioned in oncological practice guidelines for both colon and rectal carcinomas [55,56,57,58,59,60]. For a better assessment of budding degree, the team used maspin’s expression which is in the nucleus at the level of the tumor buds and helps with their identification even on the background of an abundant inflammatory stroma [56,61,62,63].
Evaluation of subcellular maspin’s expression, combined with microsatellite status, could also be of therapeutic relevance [56,64]. Cytoplasmic staining identified in serrated MSI carcinomas might indicate favorable prognosis, while nuclear expression evaluated in microsatellite stable carcinomas is associated with high-grade tumor budding, EMT, mesenchymal subtype, worse prognosis, and could indicate response to therapy with fluorouracil [54,63,64].
Proved to be related to EMT and tumor-associated angiogenesis, maspin is opening a window for potential targeted therapy [56,62,65,66,67].
3. Precision Medicine
Like other tumors, it is thought that, in the near future, the therapy of CRC will be completely based on molecular diagnostic tests. Deep learning machines can already be used for the evaluation of whole slide images and establishing histological grade, budding degree or other prognostic parameters [18].
The role of pathologist needs to be revisited and next-generation sequencing platforms will replace large parts of ancillary tests. However, as most of the molecular tests are performed from paraffin-embedded tissues, the tissue quality still depends on the pre-analytical processing. Identification of the tumor-rich areas also depends on the pathologist and its role remains crucial for proper staging and lymph node harvesting [67].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169455
References
- Laura Banias; Ioan Jung; Rebeca Chiciudean; Simona Gurzu; From Dukes-MAC Staging System to Molecular Classification: Evolving Concepts in Colorectal Cancer. International Journal of Molecular Sciences 2022, 23, 9455, 10.3390/ijms23169455.
This entry is offline, you can click here to edit this entry!
