The biogenic aliphatic polyamines (spermine, spermidine, and putrescine) are responsible for numerous cell functions, including cell proliferation, the stabilization of nucleic acid conformations, cell division, homeostasis, gene expression, and protein synthesis in living organisms. The change of polyamine concentrations in the urine or blood is usually related to the presence of malignant tumors and is regarded as a biomarker for the early diagnosis of cancer. Therefore, the detection of polyamine levels in physiological fluids can provide valuable information in terms of cancer diagnosis and in monitoring therapeutic effects.
- polyamines
- detection
- suppressor strategies
1. Polyamine Detection Methods
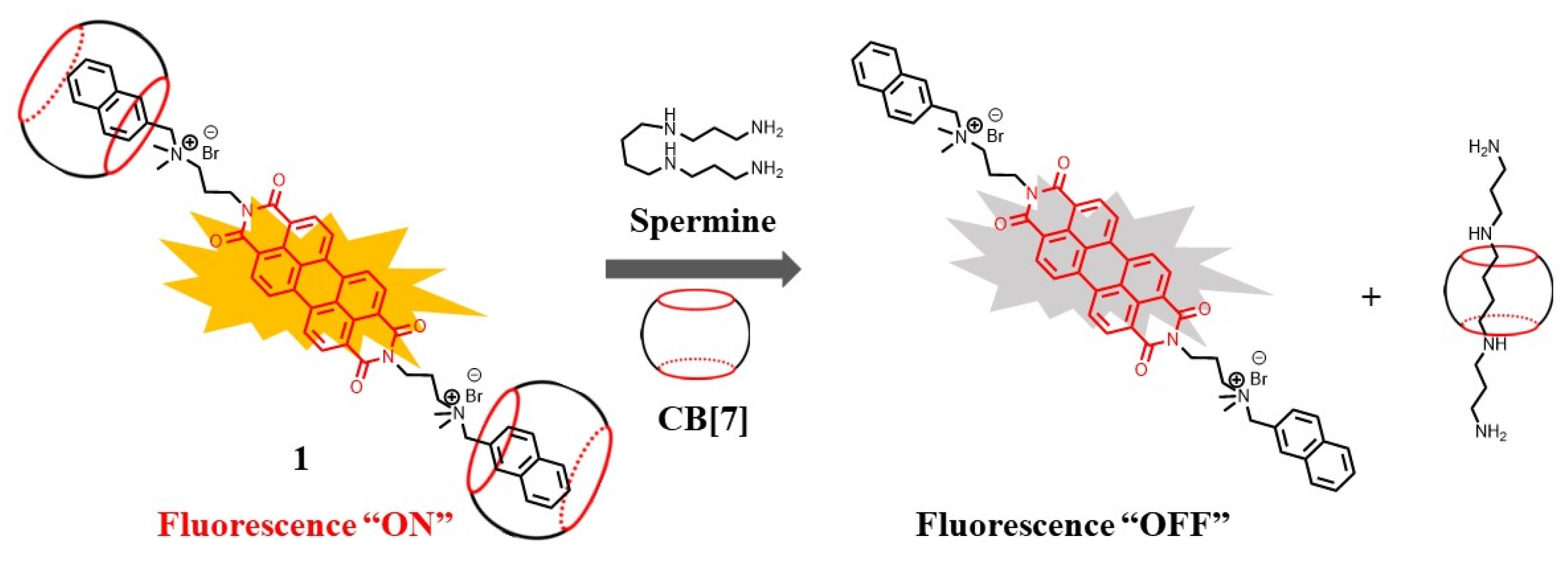
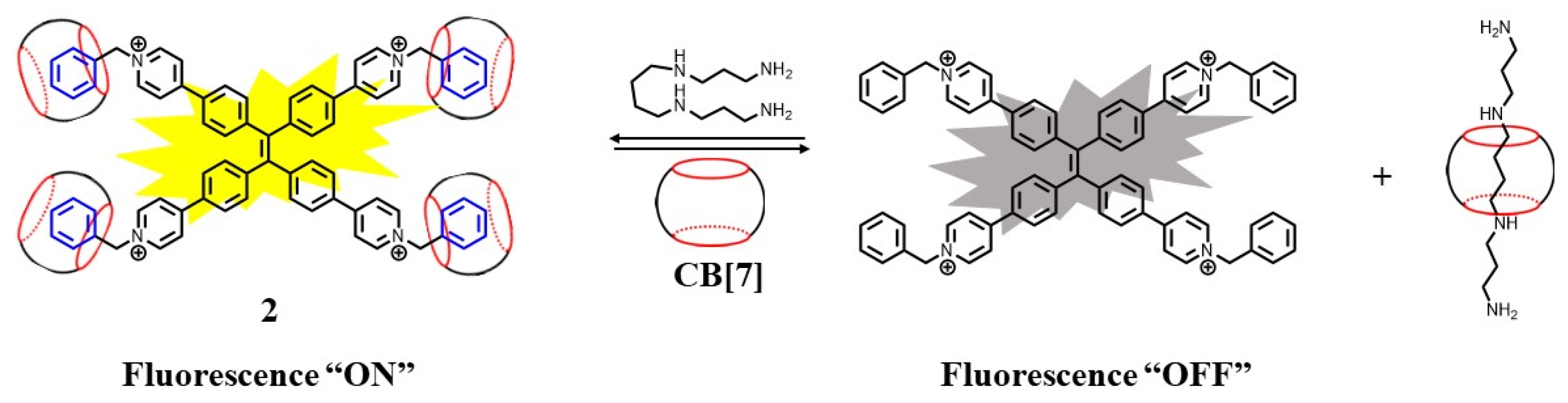
2. Supramolecular Sensing System for Polyamine Detection
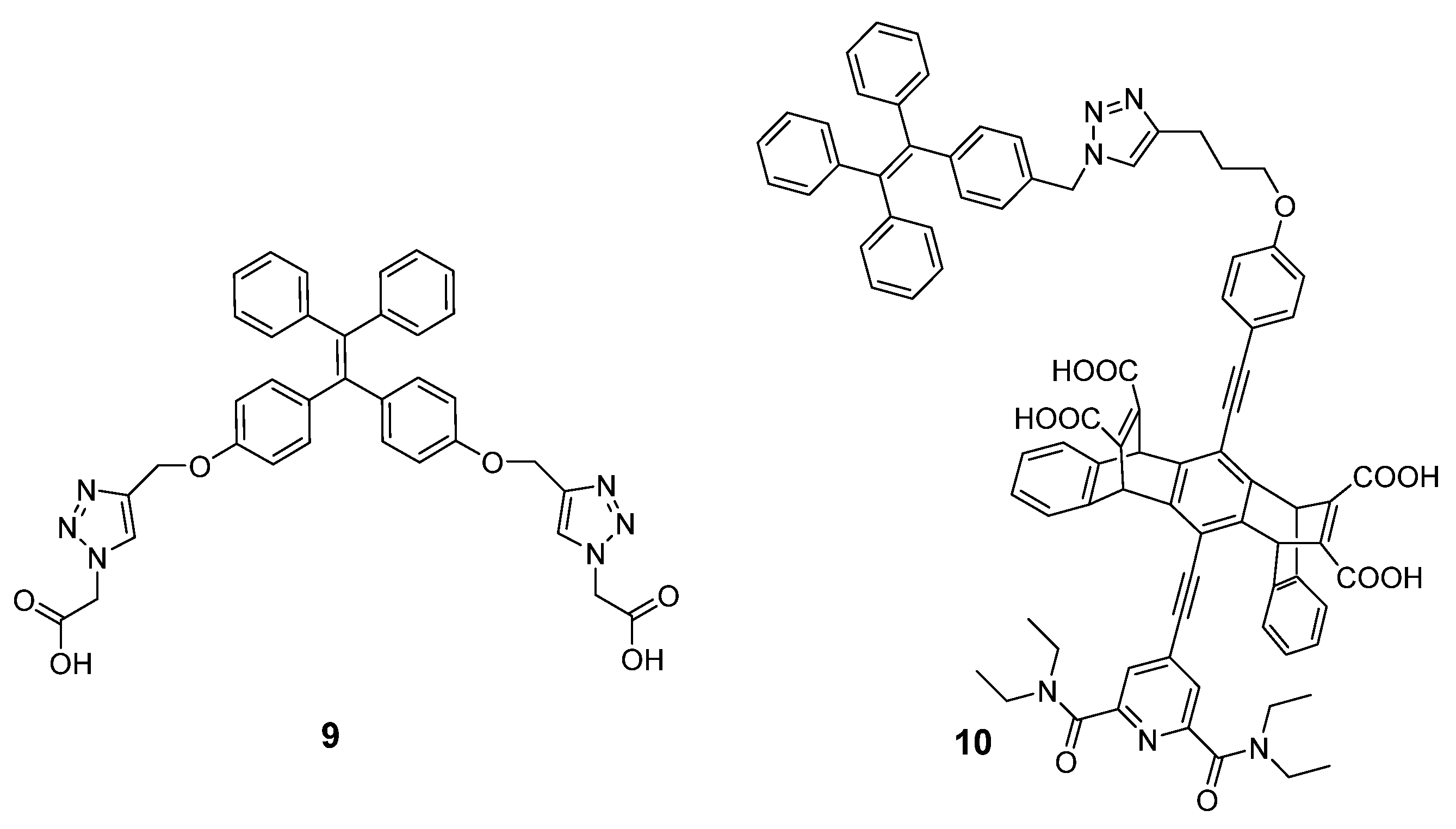
3. Polyamine Detection Based on Chromophore Reaction
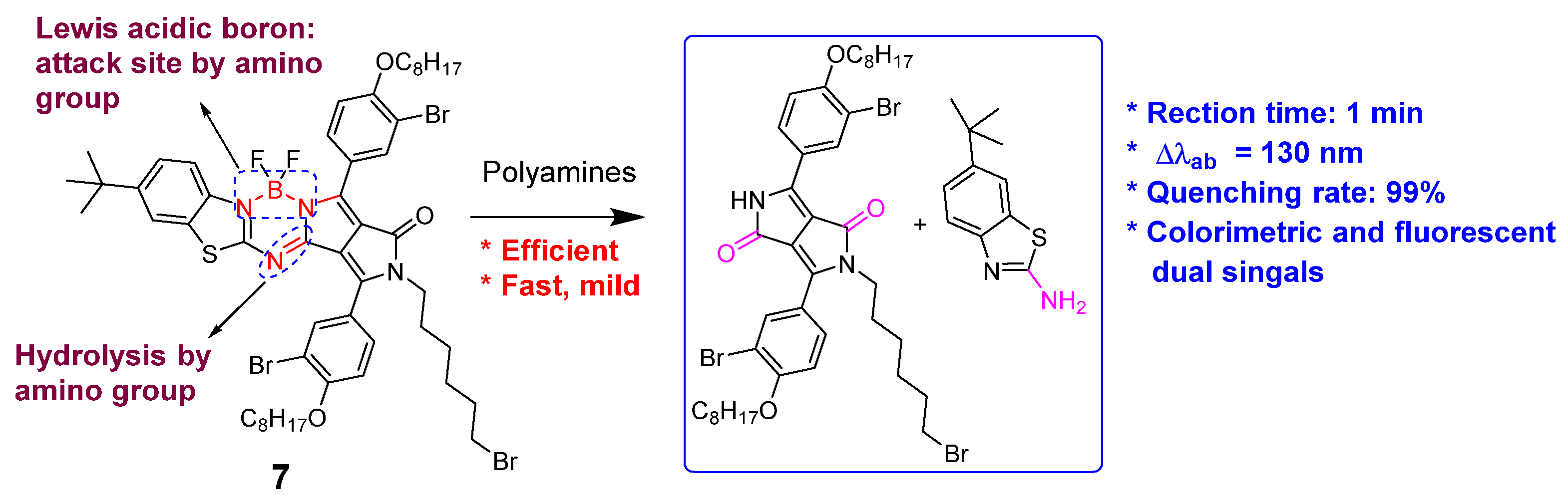
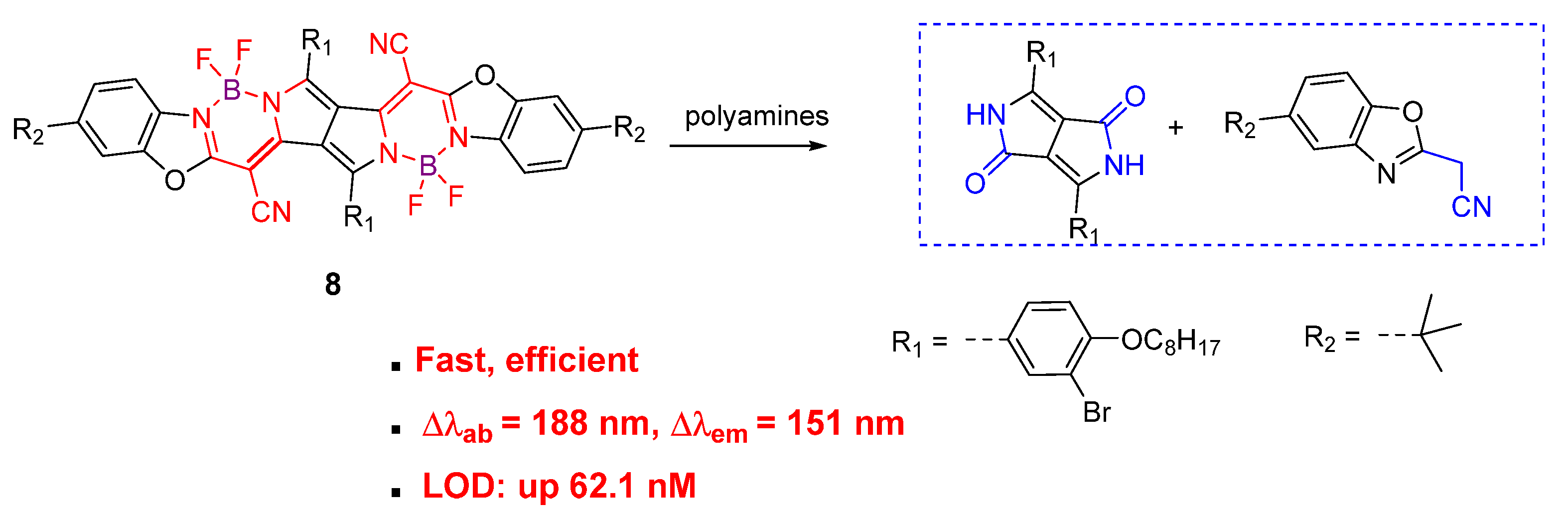
4. Fluorescent Small Molecules for Polyamine Detection
5. Fluorescent Nanoparticles for Polyamine Detection
This entry is adapted from the peer-reviewed paper 10.3390/bios12080633
References
- Matsuoka, A.; Sakamoto, T. . Rinsho Byori Jpn. J. Clin. Pathol. 2004, 52, 328–331.
- Yu, Z.; Huang, H.; Zhang, H.; Kessler, B.M. Improved Profiling of Polyamines Using Two-Dimensional Gas Chromatography Mass Spectrometry. Talanta 2019, 199, 184–188.
- Balcerzak, W.; Pokajewicz, K.; Wieczorek, P. A Useful Procedure for Detection of Polyamines in Biological Samples as a Potential Diagnostic Tool in Cancer Diagnosis. Appl. Cancer Res. 2017, 37, 23.
- Venlinen, M.; Roine, A.N.; Hkkinen, M.; Vepslinen, J.; Rantanen, T.K. Altered Polyamine Profiles in Colorectal Cancer. Anticancer Res. 2018, 38, 3601–3607.
- Takahashi, Y.; Horio, H.; Sakaguchi, K.; Hiramatsu, K.; Kawakita, M. Significant Correlation between Urinary N1, N12-Diacetylspermine and Tumor Invasiveness in Patients with Clinical Stage Ia Non-Small Cell Lung Cancer. BMC Cancer 2015, 15, 65.
- Patin, F.; Corcia, P.; Vourc’h, P.; Baranek, T.; Goossens, J.F.; Marouillat, S.; Dessein, A.F.; Descat, A.; Bruno, C.; Leman, S.; et al. Omics to Explore Amyotrophic Lateral Sclerosis Evolution: The Central Role of Arginine and Proline Metabolism. Mol. Neurobiol. 2017, 54, 5361–5374.
- Nohta, H.; Satozono, H.; Koiso, K.; Yoshida, H.; Ishida, J.; Yamaguchi, M. Highly Selective Fluorometric Determination of Polyamines Based on Intramolecular Excimer-Forming Derivatization with a Pyrene-Labeling Reagent. Anal. Chem. 2000, 72, 4199–4204.
- Lee, B.; Scopelliti, R.; Severin, K. A Molecular Probe for the Optical Detection of Biogenic Amines. Chem. Commun. 2011, 47, 9639–9641.
- Nakamura, M.; Sanji, T.; Tanaka, M. Fluorometric Sensing of Biogenic Amines with Aggregation-Induced Emission-Active Tetraphenylethenes. Chem.-Eur. J. 2011, 17, 5344–5349.
- Singh, G.; Mangat, S.; Sharma, H.; Singh, J.; Arora, A.; Singh Pannu, A.; Singh, N. Design and Syntheses of Novel Fluorescent Organosilicon-Based Chemosensors through Click Silylation: Detection of Biogenic Amines. RSC Adv. 2014, 4, 36834.
- Fletcher, J.T.; Bruck, B.S. Spermine Detection Via Metal-Mediated Ethynylarene ’Turn-on’ Fluorescence Signaling. Sens. Actuators B 2015, 207, 843–848.
- Satrijo, A.; Swager, T.M. Anthryl-Doped Conjugated Polyelectrolytes as Aggregation-Based Sensors for Nonquenching Multicationic Analytes. J. Am. Chem. Soc. 2007, 129, 16020–16028.
- Bao, B.; Yuwen, L.; Zheng, X.; Weng, L.; Zhu, X.; Zhan, X.; Wang, L. A Fluorescent Conjugated Polymer for Trace Detection of Diamines and Biogenic Polyamines. J. Mater. Chem. B 2010, 20, 9628–9634.
- Wang, J.; Zhang, Q.; Zhong, D.L.; Cheng, Z.H. Calf Thymus DNA-Stabilized Polythiophene Fluorescence Probe for Label-Free Detection of Spermine. Analyst 2012, 137, 5565–5570.
- Malik, A.H.; Hussain, S.; Iyer, P.K. Aggregation-Induced Fret Via Polymer-Surfactant Complexation: A New Strategy for the Detection of Spermine. Anal. Chem. 2016, 88, 7358–7363.
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sens. 2015, 1, 22–25.
- Ikeda, M.; Yoshii, T.; Matsui, T.; Tanida, T.; Hamachi, I. Montmorillonite-Supramolecular Hydrogel Hybrid for Fluorocolorimetric Sensing of Polyamines. J. Am. Chem. Soc. 2011, 133, 1670–1673.
- Koestereli, Z.; Severin, K. Fluorescence Sensing of Spermine with a Frustrated Amphiphile. Chem. Commun. 2012, 48, 5841–5843.
- Tu, J.; Sun, S.; Xu, Y. A Novel Self-Assembled Platform for Ratiometric Fluorescent Detection of Spermine. Chem. Commun. 2015, 52, 1040–1043.
- Chopra, S.; Singh, J.; Kaur, H.; Singh, H.; Singh, N.; Kaur, N. Selective Chemosensing of Spermidine Based on Fluorescent Organic Nanoparticles in Aqueous Media Via a Fe3+ Displacement Assay. New J. Chem. 2015, 39, 3507–3512.
- Kim, T.I.; Park, J.; Kim, Y. A Gold Nanoparticle-Based Fluorescence Turn-on Probe for Highly Sensitive Detection of Polyamines. Chem.-Eur. J. 2011, 17, 11978–11982.
- Dan, Y.; Liu, J.J.; Zhi, Z.H.; Wang, N.; Zou, H.Y.; Huang, C.Z.; Wang, J. Highly Selective Detection of Spermine in Human Urine Via a Nanometal Surface Energy Transfer Platform. Talanta 2018, 188, 218–224.
- Bhamore, J.R.; Murthy, P.; Kailasa, S.K. Fluorescence Turn-Off Detection of Spermine in Biofluids Using Pepsin Mediated Synthesis of Gold Nanoclusters as a Probe. J. Mol. Liq. 2019, 280, 18–24.
- Tawfik, S.M.; Shim, J.; Biechele-Speziale, D.; Sharipov, M.; Lee, Y.I. Novel “Turn Off-on” Sensors for Highly Selective and Sensitive Detection of Spermine Based on Heparin-Quenching of Fluorescence CdTe Quantum Dots-Coated Amphiphilic Thiophene Copolymers. Sens. Actuators B 2018, 257, 734–744.
- Zhou, Y.; Tang, H.; Li, Z.H.; Xu, L.; Wang, L.; Cao, D. Bio-Inspired AIE PillarArene Probe with Multiple Binding Sites to Discriminate Alkanediamines. Chem. Commun. 2021, 57, 13114–13117.
- Kim, Y.; Kim, T. Analyte-Directed Formation of Emissive Excimers for the Selective Detection of Polyamines. Chem. Commun. 2016, 52, 10648.
- Jiang, G.; Zhu, W.; Chen, Q.; Li, X.; Zhang, G. Selective Fluorescent Probes for Spermine and 1-Adamantanamine Based on the Supramolecular Structure Formed between AIE-Active Molecule and CucurbitUrils. Sens. Actuators B 2018, 261, 602.
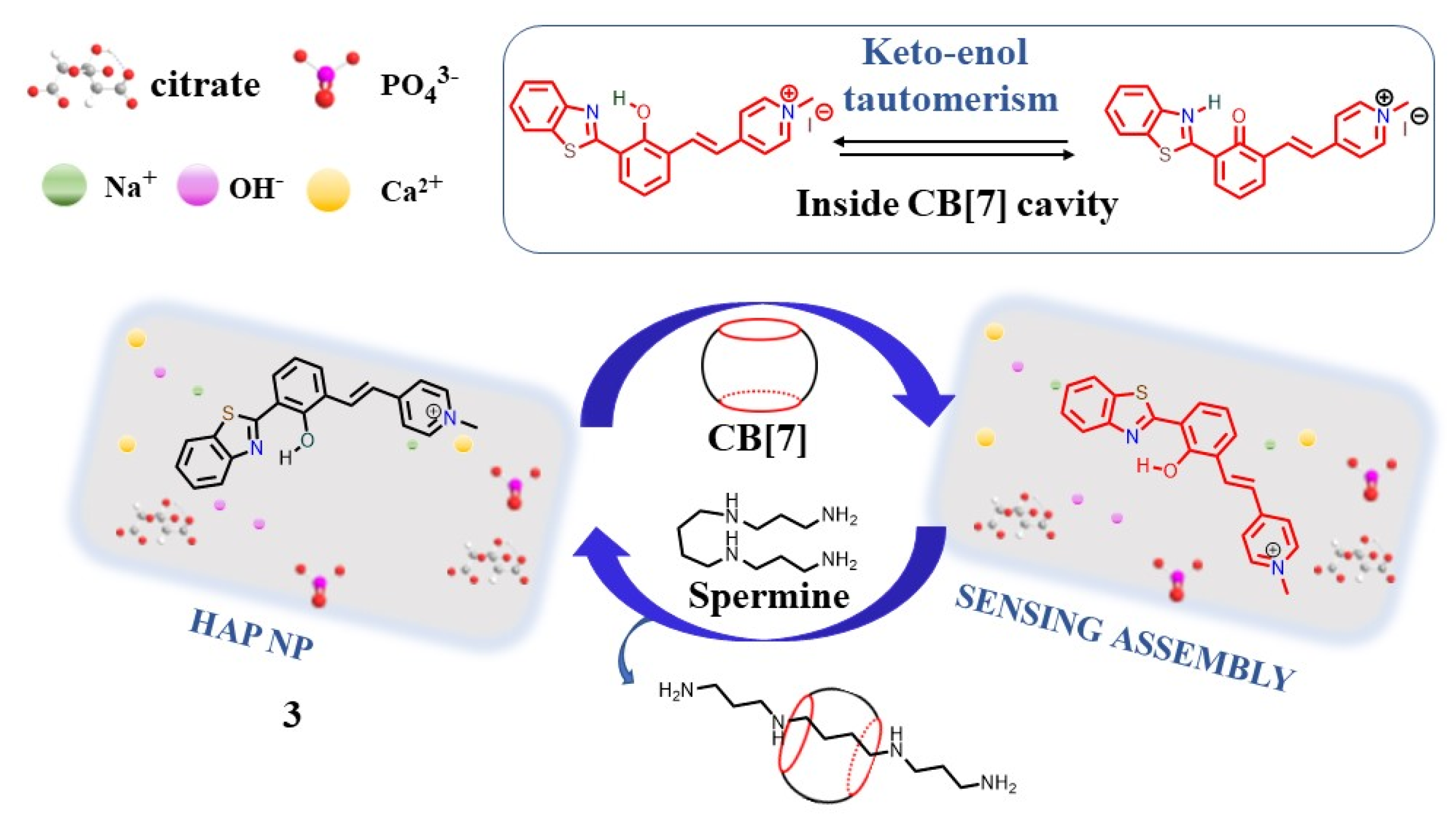
- Bhosle, A.; Banerjee, M.; Barooah, N.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Chatterjee, A. ESIPT-Active -HAP Nps Based Supramolecular Sensing Assembly for Spermine, Spermidine and Cadaverine: Application in Monitoring Cancer Biomarkers and Food Spoilage. J. Photochem. Photobiol. A 2022, 426, 113770.
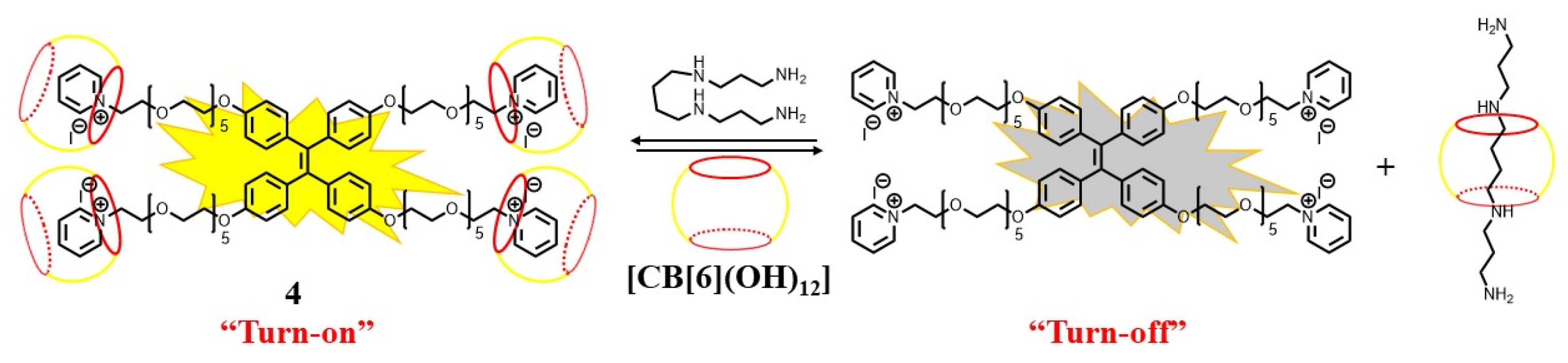
- Naik, V.G.; Kumar, V.; Bhasikuttan, A.C.; Kadu, K.; Ramanan, S.R.; Bhosle, A.A.; Banerjee, M.; Chatterjee, A. Solid-supported amplification of aggregation emission: A tetraphenylethylene–cucurbit supramolecular sensing assembly for the detection of spermine and spermidine in human urine and blood. ACS Appl. Bio Mater. 2021, 4, 1813–1822.
- Tripathi, N.; Singh, P.; Luxami, V.; Mahajan, D.; Kumar, S. Spermine Detection from Urine and Blood Serum Using Ionic Self-assembly of Benzimidazolium Based Dipod and Dodecylsulfate. Sens. Actuators B 2018, 270, 552–561.
- Nguyen, N.N.; Huy, B.T.; Phong, P.T.; Han, J.S.; Kwon, D.H.; Lee, Y. Simple Fluorescence Optosensing Probe for Spermine Based on Ciprofloxacin-Tb3+ Complexation. PLoS ONE. 2021, 16, e0251306.
- Tian, H.; Chang, Y.; Hu, X.; Li, X.; Guo, D. Supramolecular Imaging of Spermine in Cancer Cells. Nanoscale 2021, 13, 15362–15368.
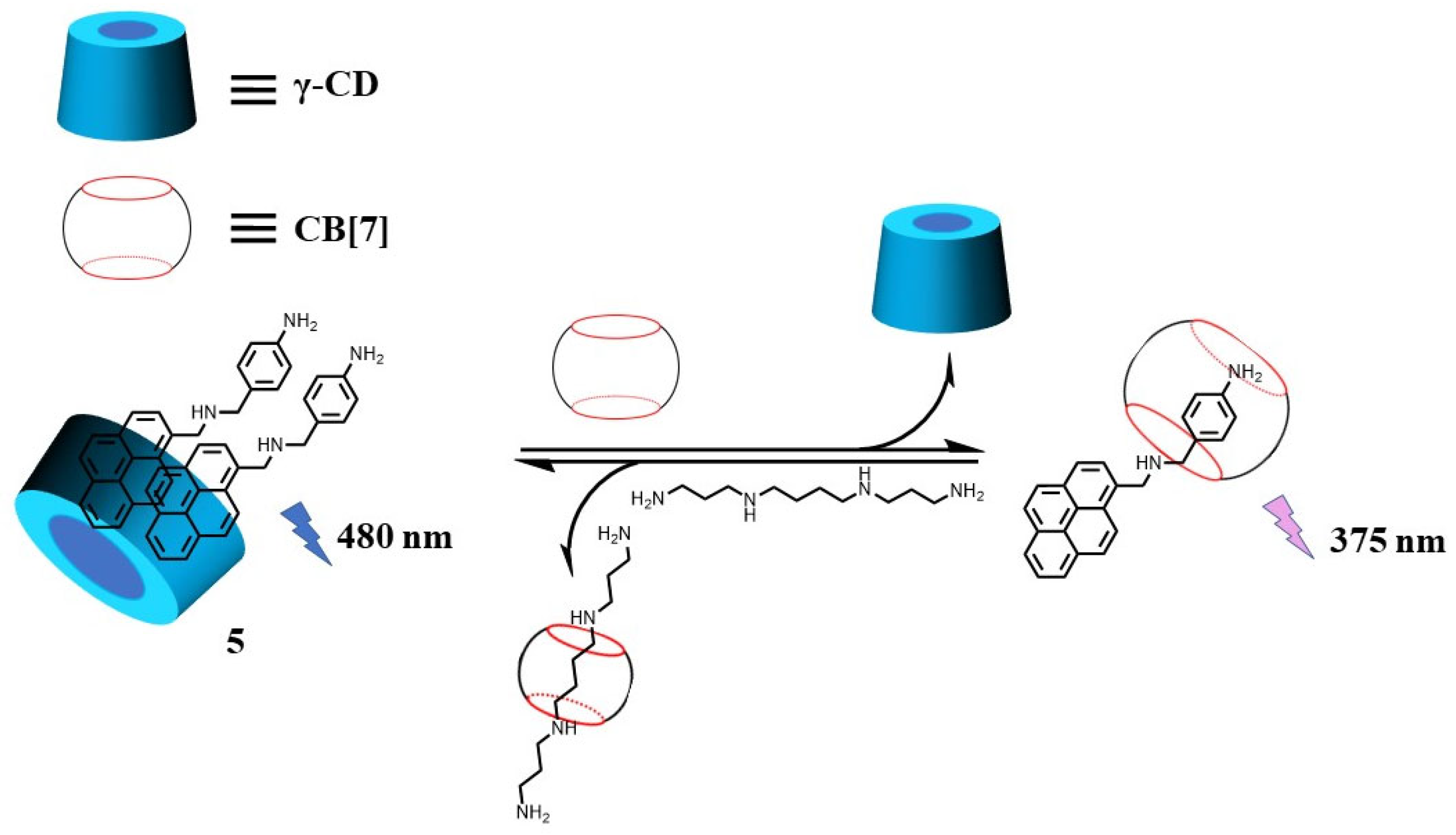
- Tian, H.; Yu, X.; Yao, J.; Gao, G.; Wu, W.; Yang, C. Supramolecular Spectral/Visual Detection of Urinary Polyamines through Synergetic/Competitive Complexation with Gamma-CD and CB. Chem. Commun. 2021, 57, 1806–1809.
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent Advances on Reaction-Based Amine Fluorescent Probes. Dyes Pigm. 2021, 194, 109634.
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent Chemodosimeters Using “Mild” Chemical Events for the Detection of Small Anions and Cations in Biological and Environmental Media. Chem. Soc. Rev. 2012, 41, 4511–4535.
- Asanuma, D.; Sakabe, M.; Kamiya, M.; Yamamoto, K.; Urano, Y. Sensitive Β-Galactosidase-Targeting Fluorescence Probe for Visualizing Small Peritoneal Metastatic Tumours in Vivo. Nat. Commun. 2015, 6, 6463.
- Uno, S.N.; Kamiya, M.; Yoshihara, T.; Sugawara, K.; Okabe, K.; Tarhan, M.C.; Fujita, H.; Funatsu, T.; Okada, Y.; Tobita, S. A Spontaneously Blinking Fluorophore Based on Intramolecular Spirocyclization for Live-Cell Super-Resolution Imaging. Nat. Chem. 2015, 6, 681–689.
- Hu, D.; Zhang, T.; Li, S.; Yu, T.; Zhang, X.; Hu, R.; Feng, J.; Wang, S.; Liang, T.; Chen, J. Ultrasensitive Reversible Chromophore Reaction of Bodipy Functions as High Ratio Double Turn on Probe. Nat. Commun. 2018, 9, 362.
- Wang, L.; Wu, S.; Tang, H.; Cao, D. An efficient probe for sensing different concentration ranges of glutathione based on AIE-active Schiff base nanoaggregates with distinct reaction mechanism. Sens. Actuators B 2018, 273, 1085–1090.
- Li, L.; Li, W.; Ran, X.; Wang, L.; Tang, H.; Cao, D. A Highly Efficient, Colorimetric and Fluorescent Probe for Recognition of Aliphatic Primary Amines Based on a Unique Cascade Chromophore Reaction. Chem. Commun. 2019, 55, 9789–9792.
- Wang, L.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Recent Advances of Nir Dyes of Pyrrolopyrrole Cyanine and Pyrrolopyrrole Aza-Bodipy: Synthesis and Application. Dyes Pigm. 2022, 198, 110040.
- Shimizu, S.; Lino, T.; Araki, Y.; Kobayashi, N. Pyrrolopyrrole Aza-Bodipy Analogues: A Facile Synthesis and Intense Fluorescence. Chem. Commun. 2013, 49, 1621–1623.
- Shimizu, S.; Iino, T.; Saeki, A.; Seki, S.; Kobayashi, N. Rational Molecular Design Towards Vis/Nir Absorption and Fluorescence by Using Pyrrolopyrrole Aza -Bodipy and Its Highly Conjugated Structures for Organic Photovoltaics. Chem.-Eur. J. 2014, 21, 2893–2904.
- Li, L.; Li, W.; Wang, L.; Tang, H.; Ran, X. Pyrrolopyrrole Aza-Bodipy Dyes for Ultrasensitive and Highly Selective Biogenic Diamine Detection. Sens. Actuators B 2020, 312, 127953.
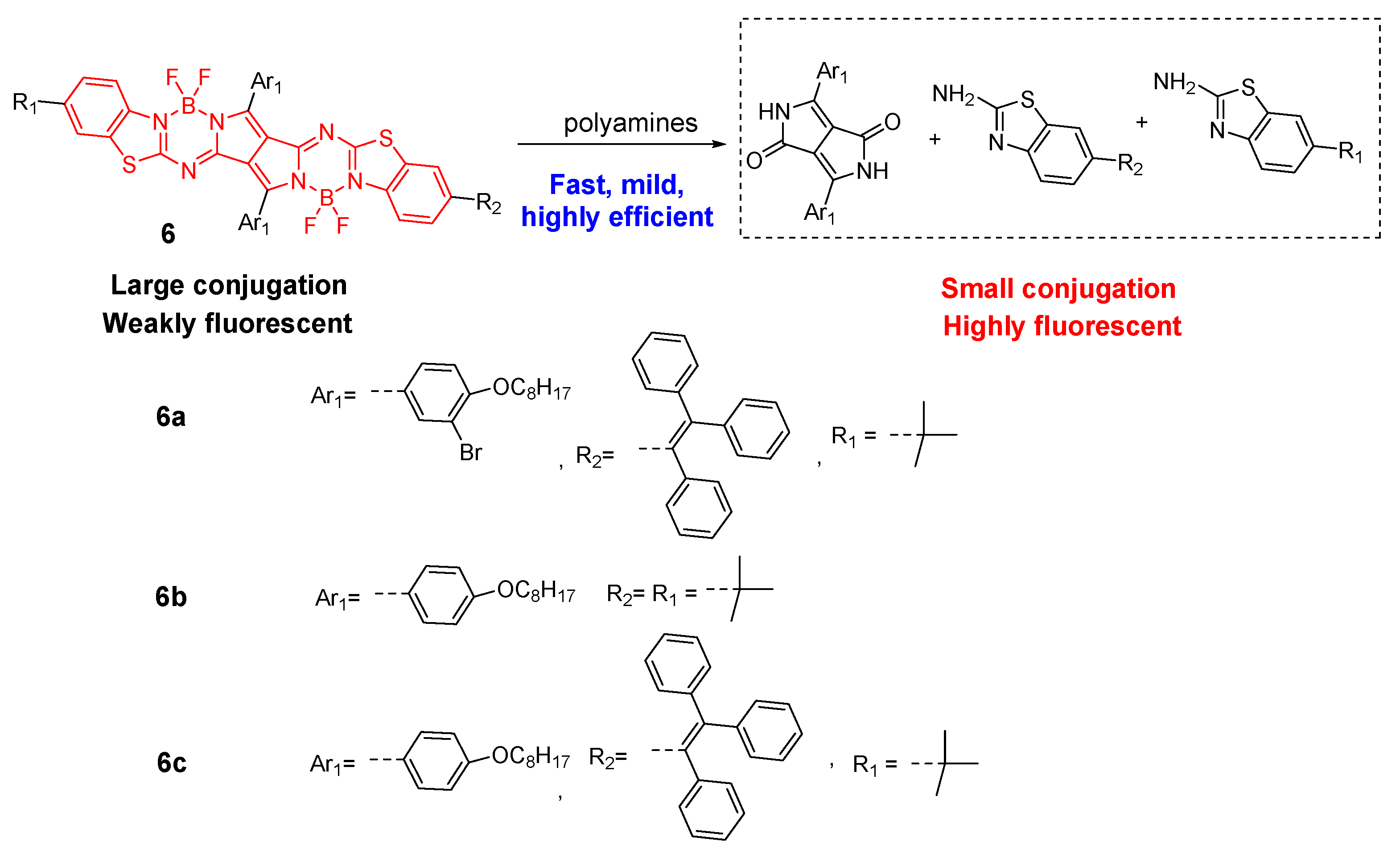
- Wang, L.; Ding, H.; Tang, H.; Cao, D.; Ran, X. A Novel and Efficient Chromophore Reaction Based on a Lactam-Fused Aza-Bodipy for Polyamine Detection. Anal. Chim. Acta 2020, 1135, 38–46.
- Wang, L.; Xin, S.; Zhang, C.; Ran, X.; Tang, H.; Cao, D. Development of a Novel Chromophore Reaction-Based Fluorescent Probe for Biogenic Amines Detection. J. Mater. Chem. B 2021, 9, 9383–9394.
- Barros, M.; Ceballos, S.; Arroyo, P.; Sáez, J.A.; Parra, M.; Gil, S.; Costero, A.M.; Gaviña, P. Spermine and Spermidine Detection through Restricted Intramolecular Rotations in a Tetraphenylethylene Derivative. Chemosensors 2022, 10, 8.
- Huang, J.; Ye, W.; Zha, S.; Tao, Y.; Yang, M.; Huang, K.; Liu, J.; Fung, J.; Li, Y.; Zhu, L.; et al. Sensitive and Responsive Pentiptycene-based Molecular Fluorescence Chemosensor for Detection of Polyamines. J. Lumin. 2021, 232, 117856.
- Samira Abbasi-Moayed, S.; Bigdeli, A.; Hormozi-Nezhad, M.R. Determination of Spermine and Spermidine in Meat with a Ratiometric Fluorescence Nanoprobe and a Combinational Logic Gate. Food Chem. 2022, 384, 132459.
- Khan, S.A.; Misra, T.K. Novel Gluconate Stabilized Gold Nanoparticles as a Colorimetric Sensor for Quantitative Evaluation of Spermine. Colloids Surf. A 2022, 648, 129146.
- Leelasree, T.; Aggarwa, H. MOF Sensors for Food Safety: Ultralow Detection of Putrescine and Cadaverine in Protein Rich Foods. J. Mater. Chem. C. 2022, 10, 2121–2127.