One of the earliest hallmarks of plant immune response is production of reactive oxygen species (ROS) in different subcellular compartments, which regulate plant immunity. A suitable equilibrium, which is crucial to prevent ROS overaccumulation leading to oxidative stress, is maintained by salicylic acid (SA), a chief regulator of ROS. However, ROS not only act downstream of SA signaling, but are also proposed to be a central component of a self-amplifying loop that regulates SA signaling as well as the interaction balance between different phytohormones. The exact role of this crosstalk, the position where SA interferes with ROS signaling and ROS interferes with SA signaling and the outcome of this regulation, depend on the origin of ROS but also on the pathosystem. The precise spatiotemporal regulation of organelle-specific ROS and SA levels determine the effectiveness of pathogen arrest and is therefore crucial for a successful immune response. However, the regulatory interplay behind still remains poorly understood, as up until now, the role of organelle-specific ROS and SA in hypersensitive response (HR)-conferred resistance has mostly been studied by altering the level of a single component. In order to address these aspects, a sophisticated combination of research methods for monitoring the spatiotemporal dynamics of key players and transcriptional activity in plants is needed and will most probably consist of biosensors and precision transcriptomics.
- plant immune response
- reactive oxygen species
- salicylic acid
- reactive oxygen species–salicylic acid crosstalk
- programmed cell death
- hypersensitive-response-conferred resistance
- RBOH NADPH oxidases
- chloroplastic redox state
- biosensors
- precision transcrip
1. Reactive Oxygen Species as One of the Earliest Hallmarks of Plant Immune Response
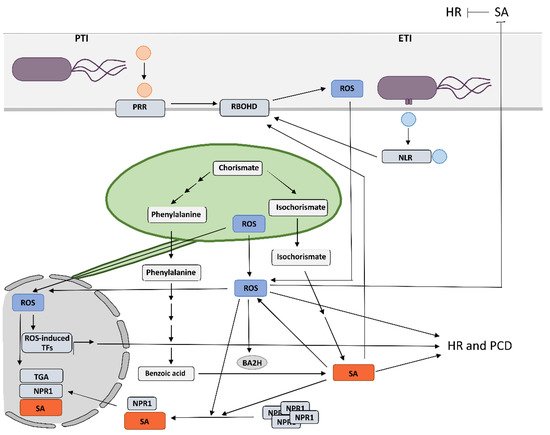
2. Crosstalk between RBOHD-Derived Reactive Oxygen Species and Salicylic Acid in Programmed Cell Death and Resistance
3. Chloroplastic Reactive Oxygen Species Play a Role in the Signaling for Programmed Cell Death and Induce SA-Dependent Transcription of Immune Genes
4. Reactive Oxygen Species of Different Source and Type Induce Diverse Transcriptional Responses
5. Tools for Studying Redox State with High Spatiotemporal Resolution with Focus on Cytoplasmic and Chloroplastic Redox State in Hypersensitive Response
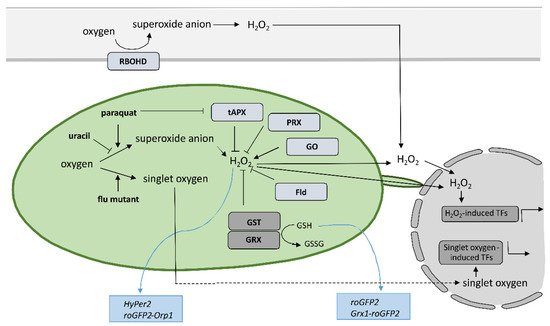
6. Conclusions
The results of the above-mentioned studies suggest that the precise spatiotemporal regulation of key players, including organelle-specific ROS and SA levels, determines the effectiveness of pathogen arrest and is therefore crucial for a successful immune response. The change of SA and ROS levels and other key players alter the rate of cell-to-cell and systemic pathogen spread, rate of cell death induction, and spatial transcriptional response, leading to susceptibility or resistance. We suggest that only a coordinated and intertwined action of all main components enable effective immune response. However, the specific interactions between them and the regulatory interplay behind still remain poorly understood, as up until now, the role of organelle-specific ROS and SA in HR-conferred resistance has only been studied by altering the level of a single component. In order to address these aspects, a sophisticated combination of research methods for monitoring the spatiotemporal dynamics of key players and transcriptional activity in plants is needed. The precise sampling of tissue sections surrounding the HR-PCD, with spatial resolution and suitable for transcriptomics analyses [16], in combination with the use of biosensors [135], could enable identification of novel key players and could unravel the interconnectivity of immune signaling components. Such an approach could therefore present a step forward in studying the resistance response.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Tsuda, K. Intimate association of PRR- and NLR-mediated signaling in plant immunity. Mol. Plant-Microbe Interact. 2021, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive—Is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, J.D.G.; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef][Green Version]
- Wang, R.; He, F.; Ning, Y.; Wang, G.L. Fine-tuning of RBOH-mediated ROS signaling in plant immunity. Trends Plant Sci. 2020, 25, 1060–1062. [Google Scholar] [CrossRef]
- Lee, D.H.; Lal, N.K.; Lin, Z.J.D.; Ma, S.; Liu, J.; Castro, B.; Toruño, T.; Dinesh-Kumar, S.P.; Coaker, G. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 2020, 11, 1838. [Google Scholar] [CrossRef][Green Version]
- Shapiguzov, A.; Julia, P.; Wrzaczek, M.; Kangasjärvi, J. ROS-talk—How the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef][Green Version]
- Borisova, M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1314–1321. [Google Scholar] [CrossRef][Green Version]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadota, Y.; Liebrand, T.W.H.; Goto, Y.; Sklenar, J.; Derbyshire, P.; Menke, F.L.H.; Torres, M.; Molina, A.; Zipfel, C.; Coaker, G.; et al. Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector- and PAMP-triggered immunity in plants. New Phytol. 2019, 221, 2160–2175. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Wang, P.Q.; Zhang, P.P.; Nie, X.M.; Li, B.B.; Tai, L.; Liu, W.T.; Li, W.Q.; Chen, K.M. NADPH oxidases: The vital performers and center hubs during plant growth and signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef][Green Version]
- Lukan, T.; Pompe-Novak, M.; Baebler, Š.; Tušek-Žnidarič, M.; Kladnik, A.; Križnik, M.; Blejec, A.; Zagorščak, M.; Stare, K.; Dušak, B.; et al. Precision transcriptomics of viral foci reveals the spatial regulation of immune-signaling genes and identifies RBOHD as an important player in the incompatible interaction between potato virus Y and potato. Plant J. 2020, 104, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Bi, Y.; Darby, R.M.; Firek, S.; Draper, J. Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersal during lesion establishment in TMV-infected tobacco. Plant J. 1997, 12, 1113–1126. [Google Scholar] [CrossRef]
- Liao, Y.; Tian, M.; Zhang, H.; Li, X.; Wang, Y.; Xia, X.; Zhou, J.; Zhou, Y.; Yu, J.; Shi, K.; et al. Salicylic acid binding of mitochondrial alpha-ketoglutarate dehydrogenase E2 affects mitochondrial oxidative phosphorylation and electron transport chain components and plays a role in basal defense against tobacco mosaic virus in tomato. New Phytol. 2015, 205, 1296–1307. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef][Green Version]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intra-cellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012, 69, 613–627. [Google Scholar] [CrossRef]
- Liu, H.B.; Wang, X.D.; Zhang, Y.Y.; Dong, J.J.; Ma, C.; Chen, W.L. NADPH oxidase RBOHD contributes to autophagy and hypersensitive cell death during the plant defense response in Arabidopsis thaliana. Biol. Plant. 2015, 59, 570–580. [Google Scholar] [CrossRef]
- Yoshie, Y.; Goto, K.; Takai, R.; Iwano, M.; Takayama, S.; Isogai, A.; Che, F.S. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnol. 2005, 22, 127–135. [Google Scholar] [CrossRef][Green Version]
- Morales, J.; Kadota, Y.; Zipfel, C.; Molina, A.; Torres, M. The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J. Exp. Bot. 2016, 67, 1663–1676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.G.; Doke, N. Nicotiana benthamiana gp91 phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; van Esse, H.P.; van Damme, M.; Fradin, E.F.; Liu, C.M.; Thomma, B.P.H.J. Ve1-mediated resistance against Verticillium does not involve a hypersensitive response in Arabidopsis. Mol. Plant Pathol. 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-induced, NADPH oxidase–derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Stone, S.; Yang, X.; Antico, J.; Callis, J.; Ramonell, K.M. ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin- and NADPH oxidase- mediated defense responses. PLoS ONE 2010, 5, e14426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Proels, R.K.; Oberhollenzer, K.; Pathuri, I.P.; Hensel, G.; Kumlehn, J.; Hückelhoven, R.; Phytopathologie, L.; München, T.U. RBOHF2 of Barley Is required for normal development of penetration resistance to the parasitic fungus Blumeria graminis f. sp. hordei. Mol. Plant-Microbe Interact. 2010, 23, 1143–1150. [Google Scholar] [CrossRef][Green Version]
- Yao, Z.; Islam, M.R.; Badawi, M.A.; El-Bebany, A.F.; Daayf, F. Overexpression of StRbohA in Arabidopsis thaliana enhances defence responses against Verticillium dahliae. Physiol. Mol. Plant Pathol. 2015, 90, 105–114. [Google Scholar] [CrossRef]
- Foley, R.C.; Gleason, C.A.; Anderson, J.P.; Hamann, T.; Singh, K.B. Genetic and genomic analysis of rhizoctonia solani interactions with arabidopsis; evidence of resistance mediated through NADPH oxidases. PLoS ONE 2013, 8, e56814. [Google Scholar] [CrossRef]
- Trujillo, M.; Altschmied, L.; Schweizer, P.; Kogel, K.H.; Hückelhoven, R. Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f. sp. hordei. J. Exp. Bot. 2006, 57, 3781–3791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pieterse, C.M.J.; van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.; Wang, Y.; Li, X.; Zhang, Y. Biosynthesis and regulation of salicylic acid and N-hydroxypipecolic acid in plant immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef][Green Version]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef][Green Version]
- Chivasa, S.; Murphy, A.M.; Naylor, M.; Carr, J.P. Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell 1997, 9, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Chivasa, S.; Carr, J.P. Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell 1998, 10, 1489–1498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Znidaric, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Zyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, C. Regulation of plant reactive oxygen species (ROS) in stress responses: Learning from AtRBOHD. Plant Cell Rep. 2016, 35, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Shirasu, K.; Moon, J.S.; Lee, S.G.; Kwon, S.Y. The activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition. PLoS ONE 2014, 9, e88951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, Y.L.; Li, W.Y.; Miao, H.; Yang, S.Q.; Li, R.; Wang, X.; Li, W.Q.; Chen, K.M. Comprehensive genomic analysis and expression profiling of the NOX gene families under abiotic stresses and hormones in plants. Genome Biol. Evol. 2016, 8, 791–810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef]
- Yoshioka, H.; Sugie, K.; Park, H.J.; Maeda, H.; Tsuda, N.; Kawakita, K.; Doke, N. Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant-Microbe Interact. 2001, 14, 725–736. [Google Scholar] [CrossRef][Green Version]
- Xi, C.; Guohui, L.; Muhammad Aamir, M.; Han, W.; Muhammad, A.; Xueqiang, S.; Jingyun, Z.; Taoshan, J.; Qing, J.; Yongping, C.; et al. In silico genome-wide analysis of respiratory burst oxidase homolog (RBOH) family genes in five fruit-producing trees, and potential functional analysis on lignification of stone cells in Chinese white pear. Cells 2019, 8, 520. [Google Scholar]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Pogány, M.; von Rad, U.; Grün, S.; Dongó, A.; Pintye, A.; Simoneau, P.; Bahnweg, G.; Kiss, L.; Barna, B.; Durner, J. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol. 2009, 151, 1459–1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Schwenkert, S.; Fernie, A.R.; Geigenberger, P.; Leister, D.; Möhlmann, T.; Naranjo, B.; Neuhaus, H.E. Chloroplasts are key players to cope with light and temperature stress. Trends Plant Sci. 2022, 27, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, M.D.; Carrillo, N.; Hajirezaei, M.R. ROS signaling in the hypersensitive response: When, where and what for? Plant Signal. Behav. 2010, 5, 393–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Ren, D.; Pike, S.; Pallardy, S.; Gassmann, W.; Zhang, S.; Life, B. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007, 51, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, M.D.; Carrillo, N.; Tognetti, V.B.; Melzer, M.; Peisker, M.; Hause, B.; Hajirezaei, M.R. Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 2009, 60, 962–973. [Google Scholar] [CrossRef]
- Straus, M.R.; Rietz, S.; ver Loren van Themaat, E.; Bartsch, M.; Parker, J.E. Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 2010, 62, 628–640. [Google Scholar] [CrossRef]
- Ishiga, Y.; Ishiga, T.; Wangdi, T.; Mysore, K.S.; Uppalapati, S.R. NTRC and chloroplast-generated reactive oxygen species regulate Pseudomonas syringae pv. tomato disease development in tomato and Arabidopsis. Mol. Plant-Microbe Interact. 2012, 25, 294–306. [Google Scholar] [CrossRef][Green Version]
- Kim, C.; Meskauskiene, R.; Zhang, S.; Lee, K.P.; Ashok, M.L.; Blajecka, K.; Herrfurth, C.; Feussner, I.; Apela, K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 2012, 24, 3026–3039. [Google Scholar] [CrossRef][Green Version]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An effector protein of the wheat stripe rust fungus targets chloroplasts and suppresses chloroplast function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [CrossRef][Green Version]
- Yao, N.; Greenberg, J.T. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 2006, 18, 397–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ochsenbein, C.; Przybyla, D.; Danon, A.; Landgraf, F.; Göbel, C.; Imboden, A.; Feussner, I.; Apel, K. The role of EDS1 (enhanced disease susceptibility) during singlet oxygen-mediated stress responses of Arabidopsis. Plant J. 2006, 47, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Lukan, T.; Županič, A.; Mahkovec Povalej, T.; Brunkard, J.O.; Juteršek, M.; Baebler, Š.; Gruden, K. Chloroplast redox state changes indicate cell-to-cell signalling during the hypersensitive response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef][Green Version]
- Noshi, M.; Maruta, T.; Shigeoka, S. Relationship between chloroplastic H2O2 and the salicylic acid response. Plant Signal. Behav. 2012, 7, 944–946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 910–926. [Google Scholar] [CrossRef][Green Version]
- Sewelam, N.; Jaspert, N.; van der Kelen, K.; Tognetti, V.B.; Schmitz, J.; Frerigmann, H.; Stahl, E.; Zeier, J.; van Breusegem, F.; Maurino, V.G. Spatial H2O2 signaling specificity: H2O2 from chloroplasts and peroxisomes modulates the plant transcriptome differentially. Mol. Plant 2014, 7, 1191–1210. [Google Scholar] [CrossRef][Green Version]
- Pierella Karlusich, J.J.; Zurbriggen, M.D.; Shahinnia, F.; Sonnewald, S.; Sonnewald, U.; Hosseini, S.A.; Hajirezaei, M.R.; Carrillo, N. Chloroplast redox status modulates genome-wide plant responses during the non-host interaction of Tobacco with the hemibiotrophic bacterium Xanthomonas campestris pv. vesicatoria. Front. Plant Sci. 2017, 8, 1158. [Google Scholar] [CrossRef]
- Kmiecik, P.; Leonardelli, M.; Teige, M. Novel connections in plant organellar signalling link different stress responses and signalling pathways. J. Exp. Bot. 2016, 67, 3793–3807. [Google Scholar] [CrossRef][Green Version]
- Pérez-Sancho, J.; Tilsner, J.; Samuels, A.L.; Botella, M.A.; Bayer, E.M.; Rosado, A. Stitching organelles: Organization and function of specialized membrane contact sites in plants. Trends Cell Biol. 2016, 26, 705–717. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanson, M.R.; Conklin, P.L. Stromules, functional extensions of plastids within the plant cell. Curr. Opin. Plant Biol. 2020, 58, 25–32. [Google Scholar] [CrossRef]
- Brunkard, J.O.; Runkel, A.M.; Zambryski, P.C. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA 2015, 112, 10044–10049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast stromules function during innate immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, A.S.; Park, E.; Nedo, A.; Alqarni, A.; Ren, L.; Hoban, K.; Modla, S.; McDonald, J.H.; Kambhamettu, C.; Dinesh-Kumar, S.P.; et al. Stromule extension along microtubules coordinated with actin-mediated anchoring guides perinuclear chloroplast movement during innate immunity. eLife 2018, 7, e23625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stonebloom, S.; Brunkard, J.O.; Cheung, A.C.; Jiang, K.; Feldman, L.; Zambryski, P. Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiol. 2012, 158, 190–199. [Google Scholar] [CrossRef][Green Version]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef][Green Version]
- He, H.; van Breusegem, F.; Mhamdi, A. Redox-dependent control of nuclear transcription in plants. J. Exp. Bot. 2018, 69, 3359–3372. [Google Scholar] [CrossRef]
- Locato, V.; Cimini, S.; de Gara, L. ROS and redox balance as multifaceted players of cross-tolerance: Epigenetic and retrograde control of gene expression. J. Exp. Bot. 2018, 69, 3373–3391. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef][Green Version]
- Bleau, J.R.; Spoel, S.H. Selective redox signaling shapes plant-pathogen interactions. Plant Physiol. 2021, 186, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N.; Park, E. Spatial chloroplast-to-nucleus signalling involving plastid–nuclear complexes and stromules. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190405. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Karpinska, B.; Krupinska, K. The functions of WHIRLY1 and REDOXRESPONSIVE TRANSCRIPTION FACTOR 1 in cross tolerance responses in plants: A hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grabowski, E.; Miao, Y.; Mulisch, M.; Krupinska, K. Single-stranded DNA-binding protein whirly1 in barley leaves is located in plastids and the nucleus of the same cell. Plant Physiol. 2008, 147, 1800–1804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, X.; Feng, P.; Xu, X.; Guo, H.; Ma, J.; Chi, W.; Lin, R.; Lu, C.; Zhang, L. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011, 2, 477. [Google Scholar] [CrossRef][Green Version]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Villasante, C.; Burén, S.; Blázquez-Castro, A.; Barón-Sola, Á.; Hernández, L.E. Fluorescent in vivo imaging of reactive oxygen species and redox potential in plants. Free Radic. Biol. Med. 2018, 122, 202–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, W.G.; Swanson, S.J.; Gilroy, S. High-resolution imaging of Ca 2+, redox status, ROS and pH using GFP biosensors. Plant J. 2012, 70, 118–128. [Google Scholar] [CrossRef]
- Fichman, Y.; Miller, G.; Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar] [CrossRef][Green Version]
- Jiang, K.; Schwarzer, C.; Lally, E.; Zhang, S.; Ruzin, S.; Machen, T.; Remington, S.J.; Feldman, L. Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol. 2006, 141, 397–403. [Google Scholar] [CrossRef][Green Version]
- Rosenwasser, S.; Rot, I.; Meyer, A.J.; Feldman, L.; Jiang, K.; Friedman, H. A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiol. Plant. 2010, 138, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Schwarzländer, M.; Fricker, M.D.; Müller, C.; Marty, L.; Brach, T.; Novak, J.; Sweetlove, L.J.; Hell, R.; Meyer, A.J. Confocal imaging of glutathione redox potential in living plant cells. J. Microsc. 2008, 231, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J.; Brach, T.; Marty, L.; Kreye, S.; Rouhier, N.; Jacquot, J.P.; Hell, R. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007, 52, 973–986. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Fricker, M.D.; Sweetlove, L.J. Monitoring the in vivo redox state of plant mitochondria: Effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim. Biophys. Acta-Bioenerg. 2009, 1787, 468–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haber, Z.; Lampl, N.; Meyer, A.J.; Zelinger, E.; Hipsch, M.; Rosenwasser, S. Resolving diurnal dynamics of the chloroplastic glutathione redox state in Arabidopsis reveals its photosynthetically derived oxidation. Plant Cell 2021, 33, 1828–1844. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B.N.; Foley, R.; Singh, K.B.; Anderson, J.P. Foliar resistance to Rhizoctonia solani in Arabidopsis is compromised by simultaneous loss of ethylene, jasmonate and PEN2 mediated defense pathways. Sci. Rep. 2021, 11, 2546. [Google Scholar] [CrossRef] [PubMed]
- Hipsch, M.; Lampl, N.; Zelinger, E.; Barda, O.; Waiger, D.; Rosenwasser, S. Sensing stress responses in potato with whole-plant redox imaging. Plant Physiol. 2021, 187, 618–631. [Google Scholar] [CrossRef]
- Nietzel, T.; Elsässer, M.; Ruberti, C.; Steinbeck, J.; Ugalde, J.M.; Fuchs, P.; Wagner, S.; Ostermann, L.; Moseler, A.; Lemke, P.; et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 2019, 221, 1649–1664. [Google Scholar] [CrossRef][Green Version]
- Fuchs, R.; Kopischke, M.; Klapprodt, C.; Hause, G.; Meyer, A.J.; Schwarzländer, M.; Fricker, M.D.; Lipka, V. Immobilized subpopulations of leaf epidermal mitochondria mediate PENETRATION2-dependent pathogen entry control in arabidopsis. Plant Cell 2016, 28, 130–145. [Google Scholar] [CrossRef][Green Version]
- Maughan, S.C.; Pasternak, M.; Cairns, N.; Kiddle, G.; Brach, T.; Jarvis, R.; Haas, F.; Nieuwland, J.; Lim, B.; Müller, C.; et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2331–2336. [Google Scholar] [CrossRef][Green Version]
- Krönauer, C.; Lahaye, T. The flavin monooxygenase Bs3 triggers cell death in plants, impairs growth in yeast and produces H2O2 in vitro. PLoS ONE 2021, 16, e0256217. [Google Scholar] [CrossRef] [PubMed]
- Gutscher, M.; Pauleau, A.L.; Marty, L.; Brach, T.; Wabnitz, G.H.; Samstag, Y.; Meyer, A.J.; Dick, T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 2008, 5, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Marty, L.; Siala, W.; Schwarzländer, M.; Fricker, M.D.; Wirtz, M.; Sweetlove, L.J.; Meyer, Y.; Meyer, A.J.; Reichheld, J.P.; Hell, R. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 9109–9114. [Google Scholar] [CrossRef][Green Version]
- Albrecht, S.C.; Sobotta, M.C.; Bausewein, D.; Aller, I.; Hell, R.; Dick, T.P.; Meyer, A.J. Redesign of genetically encoded biosensors for monitoring mitochondrial redox status in a broad range of model eukaryotes. J. Biomol. Screen. 2014, 19, 379–386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aller, I.; Rouhier, N.; Meyer, A.J. Development of roGFP2-derived redox probes for measurement of the glutathione redox potential in the cytosol of severely glutathione-deficient rml1 seedlings. Front. Plant Sci. 2013, 4, 506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F. Lo H2O2 in plant peroxisomes: An in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 2010, 62, 760–772. [Google Scholar] [CrossRef][Green Version]
- Ugalde, J.M.; Schlößer, M.; Dongois, A.; Martinière, A.; Meyer, A.J. The latest HyPe(r) in plant H2O2 biosensing. Plant Physiol. 2021, 187, 480–484. [Google Scholar] [CrossRef]
- Dubreuil-Maurizi, C.; Poinssot, B. Role of glutathione in plant signaling under biotic stress. Plant Signal. Behav. 2012, 7, 210–212. [Google Scholar] [CrossRef][Green Version]
- Dubreuil-Maurizi, C.; Vitecek, J.; Marty, L.; Branciard, L.; Frettinger, P.; Wendehenne, D.; Meyer, A.J.; Mauch, F.; Poinssot, B. Glutathione deficiency of the Arabidopsis mutant pad2-1 affects oxidative stress-related events, defense gene expression, and the hypersensitive response. Plant Physiol. 2011, 157, 2000–2012. [Google Scholar] [CrossRef][Green Version]
- Matern, S.; Peskan-Berghoefer, T.; Gromes, R.; Kiesel, R.V.; Rausch, T. Imposed glutathione-mediated redox switch modulates the tobacco wound-induced protein kinase and salicylic acid-induced protein kinase activation state and impacts on defence against Pseudomonas syringae. J. Exp. Bot. 2015, 66, 1935–1950. [Google Scholar] [CrossRef][Green Version]
- Hussain, J.; Chen, J.; Locato, V.; Sabetta, W.; Behera, S.; Cimini, S.; Griggio, F.; Martínez-Jaime, S.; Graf, A.; Bouneb, M.; et al. Constitutive cyclic GMP accumulation in Arabidopsis thaliana compromises systemic acquired resistance induced by an avirulent pathogen by modulating local signals. Sci. Rep. 2016, 6, 36423. [Google Scholar] [CrossRef] [PubMed]
- Doccula, F.G.; Luoni, L.; Behera, S.; Bonza, M.C.; Costa, A. In vivo analysis of calcium levels and glutathione redox status in Arabidopsis epidermal leaf cells infected with the hypersensitive response-inducing bacteria pseudomonas. Methods Mol. Biol. 2018, 1743, 125–141. [Google Scholar] [PubMed]
- Mencia, R.; Céccoli, G.; Fabro, G.; Torti, P.; Colombatti, F.; Ludwig-Müller, J.; Alvarez, M.E.; Welchen, E. OXR2 increases plant defense against a hemibiotrophic pathogen via the salicylic acid pathway. Plant Physiol. 2020, 184, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, J.M.; Fuchs, P.; Nietzel, T.; Cutolo, E.A.; Homagk, M.; Vothknecht, U.C.; Holuigue, L.; Schwarzländer, M.; Müller-Schüssele, S.J.; Meyer, A.J. Chloroplast-derived photo-oxidative stress causes changes in H2O2 and EGSH in other subcellular compartments. Plant Physiol. 2021, 186, 125–141. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 4. [Google Scholar] [CrossRef][Green Version]
- Kostyuk, A.I.; Panova, A.S.; Kokova, A.D.; Kotova, D.A.; Maltsev, D.I.; Podgorny, O.V.; Belousov, V.V.; Bilan, D.S. In vivo imaging with genetically encoded redox biosensors. Int. J. Mol. Sci. 2020, 21, 8164. [Google Scholar] [CrossRef]
- Sugiura, K.; Yokochi, Y.; Fu, N.; Fukaya, Y.; Yoshida, K.; Mihara, S.; Hisabori, T. The thioredoxin (Trx) redox state sensor protein can visualize Trx activities in the light/dark response in chloroplasts. J. Biol. Chem. 2019, 294, 12091–12098. [Google Scholar] [CrossRef]
- Ortega-Villasante, C.; Burén, S.; Barón-Sola, Á.; Martínez, F.; Hernández, L.E. In vivo ROS and redox potential fluorescent detection in plants: Present approaches and future perspectives. Methods 2016, 109, 92–104. [Google Scholar] [CrossRef][Green Version]
- Dogra, V.; Kim, C. Singlet oxygen metabolism: From genesis to signaling. Front. Plant Sci. 2020, 10, 1640. [Google Scholar] [CrossRef][Green Version]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Awad, J.; Stotz, H.U.; Fekete, A.; Krischke, M.; Engert, C.; Havaux, M.; Berger, S.; Mueller, M.J. 2-cysteine peroxiredoxins and thylakoid ascorbate peroxidase create awater-water cycle that is essential to protect the photosynthetic apparatus under high light stress conditions. Plant Physiol. 2015, 167, 1592–1603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murgia, I.; Tarantino, D.; Vannini, C.; Bracale, M.; Carravieri, S.; Soave, C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004, 38, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Palatnik, J.F.; Fillat, M.F.; Melzer, M.; Hajirezaei, M.R.; Valle, E.M.; Carrillo, N. Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 2006, 18, 2035–2050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tognetti, V.B.; Zurbriggen, M.D.; Morandi, E.N.; Fillat, M.F.; Valle, E.M.; Hajirezaei, M.R.; Carrillo, N. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 11495–11500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zurbriggen, M.D.; Tognetti, V.B.; Fillat, M.F.; Hajirezaei, M.R.; Valle, E.M.; Carrillo, N. Combating stress with flavodoxin: A promising route for crop improvement. Trends Biotechnol. 2008, 26, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Coba de la Peña, T.; Redondo, F.J.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol. J. 2010, 8, 954–965. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, S.; Jia, H.; Gao, F.; Zhou, M.; Yuan, N.; Wu, P.; Hu, Q.; Sun, D.; Luo, H. Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol. J. 2017, 15, 433–446. [Google Scholar] [CrossRef]
- Pierella Karlusich, J.J.; Arce, R.C.; Shahinnia, F.; Sonnewald, S.; Sonnewald, U.; Zurbriggen, M.D.; Hajirezaei, M.R.; Carrillo, N. Transcriptional and metabolic profiling of potato plants expressing a plastid-targeted electron shuttle reveal modulation of genes associated to drought tolerance by chloroplast redox poise. Int. J. Mol. Sci. 2020, 21, 7199. [Google Scholar] [CrossRef]
- Fahnenstich, H.; Scarpeci, T.E.; Valle, E.M.; Flügge, U.I.; Maurino, V.G. Generation of hydrogen peroxide in chloroplasts of arabidopsis overexpressing glycolate oxidase as an inducible system to study oxidative stress. Plant Physiol. 2008, 148, 719–729. [Google Scholar] [CrossRef][Green Version]
- Schmidt, A.; Mächtel, R.; Ammon, A.; Engelsdorf, T.; Schmitz, J.; Maurino, V.G.; Voll, L.M. Reactive oxygen species dosage in Arabidopsis chloroplasts can improve resistance towards Colletotrichum higginsianum by the induction of WRKY33. New Phytol. 2020, 226, 189–204. [Google Scholar] [CrossRef][Green Version]
- Meskauskiene, R.; Nater, M.; Goslings, D.; Kessler, F.; Op den Camp, R.; Apel, K. FLU: A negative regulator of chlorophyll biosynthesis in arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 12826–12831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scarpeci, T.E.; Zanor, M.I.; Carrillo, N.; Mueller-Roeber, B.; Valle, E.M. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: A focus on rapidly induced genes. Plant Mol. Biol. 2008, 66, 361–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mano, J.; Ohno, C.; Domae, Y.; Asada, K. Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: Its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim. Biophys. Acta—Bioenerg. 2001, 1504, 275–287. [Google Scholar] [CrossRef][Green Version]
- Abdollahi, H.; Ghahremani, Z. The role of chloroplasts in the interaction between Erwinia amylovora and host plants. Acta Hortic. 2011, 896, 215–222. [Google Scholar] [CrossRef]
- Levak, V.; Lukan, T.; Gruden, K.; Coll, A. Biosensors: A sneak peek into plant cell’s immunity. Life 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed]
This entry is adapted from the peer-reviewed paper 10.3390/ijms23105568
