Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The emergence of COVID-19, caused by the SARS-CoV-2 virus, led to the pursuit of solutions for the treatment of symptoms and/or disease. Honey has proven to be effective against viral infections, principally due to its potential antioxidant and anti-inflammatory activities that attenuate oxidative damage induced by pathogens, and by improving the immune system.
- honey
- COVID-19
- natural products
- biological properties
1. Introduction
The value of honey is recognized all over the world. Several studies have been performed in different fields concerning honey and its related products. These natural products have effects on the environment, biodiversity, and human health assurance. The role of honey in health and wellbeing is well recognized, with its medicinal and nutritious performance being extremely explored over human history. In recent years, concerns about the environment and the preservation of the planet have increased. This had led to a search for natural products as alternatives to processed foods and chemical foodstuffs. In this sense, honey, and its related products, such as bee pollen, beeswax, beebread, and propolis, are natural products favored by consumers and industries. Based on their biological properties, these products have been selected for a wide range of applications, as ingredient or as a single product, to address old and new problems. Honey’s biological, nutraceutical and medicinal properties have been scientifically proven, especially regarding antioxidant, anti-inflammatory, antibacterial and antidiabetic activities, as well as for respiratory, gastrointestinal, cardiovascular, and nervous system protective effects [1][2][3]. The antibacterial activity of honey is one of its most valued properties. In fact, honey is increasingly used in its pure form, or in mixtures with drugs, for the treatment of infections, burns and wounds. Its antibacterial power is mainly attributed to its high osmolarity, acidity (low pH), and content of hydrogen peroxide (H2O2) and non-peroxide components [4][5]. Consequently, different studies have been increasingly performed, involving honey and related products, aiming to understand and prove the real health values of this natural product [6][7]. Despite the high nutritional and medical value attributed to the generality of honey products, there are differences among them that may result in different attributes and properties. Honey can be classified as blossom or nectar honey: the first one is produced by honeybees from the nectar of plants, while the second result from secretions of living parts of plants or excretions of plant-sucking insects on the living parts of plants [3][8]. Additionally, nectar honey can be classified as unifloral or multifloral, depending on its predominant production from a single, or from several plant species, respectively. Unifloral honey is considered high-quality honey, since it can attain not only specific flavor and organoleptic properties, but also specific biological properties. Despite its essential composition of water and sugars (mainly fructose and glucose), honey also contains other minor compounds, namely vitamins, minerals, enzymes, free amino acids, and numerous volatile compounds [3][9][10]. These compounds are responsible for conferring specific/individual organoleptic, nutritional, and biological properties, depending on the botanical origin, geographic area, season, and technology used for honey extraction, as well as storage conditions [3].
2. Honey as a Co-Adjuvant of SARS-CoV-2 Infection Treatment
Honey and its related products are used as natural therapies for several health problems, including pulmonary and cardiovascular disorders, diabetes, hypertension, gastrointestinal tract disorders, edema, cancer, autophagy dysfunction, bacterial, viral, and fungal infections [11][12]. These biological properties are principally due to honey’s potential antioxidant and anti-inflammatory activities, which may not only attenuate the oxidative damage induced by pathogens but also improve the immune system. In the specific case of viral infections, honey is employed due to its abilities to decrease acute inflammation by promoting an immune response [13][14]. In fact, honey can modulate the molecular targets involved in cellular signaling pathways, such as apoptosis and inflammation, as well as signaling cascades needed for virus replication and attachment to the host cells [15][16]. Varicella zoster [17], rubella [18], influenza [19], herpes simplex [20], respiratory syncytial virus [21], immunodeficiency virus [22], viral hepatitis A [22], are among the pathogens effectively inhibited by honey.
Honey is mainly composed of sugars and water, while minor compounds such as organic acids, amino acids, enzymes, phenolic compounds, vitamins, minerals, and antioxidants, are related with its biological properties [23][24][25][26][27]. The foraging activity of honeybees during honey production can lead to variations in honey composition, and are highly dependent of the plant species visited. Additionally, seasonal, environmental, and processing factors, as well as genus and bee species, may also influence honey composition and its biological effects [28][29]. Some research has suggested that many of the therapeutic properties of plants can be transmitted to honey, being a transport vector for the plant’s medicinal properties [30]. Due to its composition and biological properties, honey and its related products are applied directly, or as components, in the treatment of several diseases. These products have proven their virucidal effect on several enveloped viruses such as HIV, influenza virus, herpes simplex, and varicella-zoster virus [17][19][31][32].
Among the different biological properties of honey, the most reported is its antioxidant activity. It is well known that antioxidants can prevent cell death by draining lymphocytes, which leads to antiviral action [26][33][34]. Corrêa and Rogero [35] observed that a polyphenol-rich environment stimulates the activation of the immune system and the mechanisms involved in tissue repair. Manuka honey, a monofloral honey derived from the nectar of the manuka tree (Leptospermum scoparium), an indigenous plant of New Zealand, has greatly attracted the attention of researchers due to its biological properties; especially its antimicrobial and antioxidant capacities [11][34]. The chemical composition of Manuka honey includes an abundant suite of polyphenols and other bioactive compounds such as glyoxal and methylglyoxal (MGO), methyl syringate, and leptosis [11][34]. Particularly, the α-ketoaldehyde compound, MGO, can inhibit growth of the enveloped virus [19] and, together with the antiseptic effect of hydrogen peroxide, this aldehyde increases the antibacterial effect reported for this honey [25]. Combarros-Fuertes et al. [34] analyzed 16 different honey samples to select the best one for therapeutic purposes. The antioxidant activity, the main bioactive compounds and the phenolic profiles were determined. The samples exhibited great variability, with values ranging between 0.34 and 75.8 mg/100 g honey for ascorbic acid, 23.1 to 158 mg of equivalents of gallic acid/100 g honey for total phenolics content (TPC) and between 1.65 and 5.93 mg equivalents of catechin/100 g honey for the total flavonoid content (TFC). Forty-nine different phenolic compounds were detected. The concentration of the phenolic compounds and their phenolic profiles varied extensively among samples (ranging from 1.06 to 18.6 mg/100 g honey). The same was observed regarding antioxidant activity. Although the samples with better combination of bioactive properties were avocado and chestnut honeys, all of them had great antibacterial activity.
Different in vitro and in vivo studies have supported that flavonoids can inhibit Angiotensin-Converting enzyme (ACE), the target binding receptor of the SARS-CoV protein, indicating that honey and related products could exhibit a marked activity for COVID-19 treatment [14][36][37][38]. However, these conclusions must be supported by experimental studies. Moreover, due to the presence of certain compounds, such as methylglyoxal (MGO), copper, ascorbic acid, flavonoids, nitric oxide, hydrogen peroxide, and its derivatives, honey can suppress viral growth by inhibiting viral replication and/or virucidal activity [19][33].
The bioactivity of honey against SARS-CoV-2 infection is mainly driven by three mechanisms (Figure 1): (a) direct virucidal properties; (b) regulation/boost of host immune signaling pathways, and (c) cure and/or improvement of comorbid conditions.
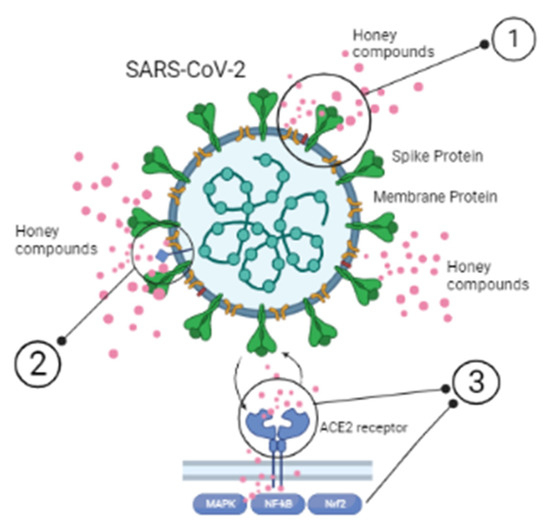
Figure 1. Possible mechanisms responsible for honey bioactivity against SARS-CoV-2: (1) altering the viral structure: interaction of honey and its major components with structural and/or non-structural proteins in the virus; (2) binding to target receptors on the virus; (3) interrupting membrane proteins (crucial for the viral attachment and entry into the host cells). Illustrations were made with BioRender.
2.1. Direct Virucidal Properties
The antiviral activity of honey may occur by different mechanisms. According to different researchers, honey can inhibit viral infection by altering the structure of the surface protein, binding to target receptors on the virus, or interrupting the membrane proteins crucial for viral attachment and entry into the host cells [15][16]. Moreover, due to the presence of several compounds, such as MGO, copper, ascorbic acid, flavonoids, nitric oxide, hydrogen peroxide, and its derivatives, honey can suppress viral growth by inhibiting viral replication and/or virucidal activity [39]. Recently, a review focused on in silico, in vitro and clinical studies of honey and propolis effects on COVID-19, highlighted the positive effect of flavonoids, such as, rutin, naringin, and quercetin, on treatment against SARS-CoV-2. The trace element copper is a well-known inactivator of viruses, while phenolic compounds, ascorbic acid and hydrogen peroxide inhibit viral growth by disrupting viral transcription, translation, and replication [39]. Regarding hydrogen peroxide, routine daily honey intake might provide protection against SARS-CoV-2 due to the biocidal effect of this reactive oxygen species, helping to clean the throat from virus particles. Moreover, the physicochemical properties of honey, namely pH (that ranges between 3.5 and 4.5), osmolarity, viscosity, and thickness, can also contribute to the antimicrobial effects reported [40].
2.2. Regulation/Boosting of Host Immune Signaling Pathways
Oxidative stress has a role in several pathological conditions, including neurological disorders, cancer, aging, endocrine illness, and pathogen infection. During virus invasion, oxidative stress induces inflammatory damage, with a consequently exacerbated immune response, the so-called cytokine storm [33]. Most SARS-CoV-2 patients showed elevated serum levels of C reactive protein (CRP), a marker of systemic inflammation [41]. The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is generally counterbalanced by the action of antioxidant molecules or enzymes. The antioxidant effect of honey [13][16][42] is mainly correlated with the content of phenolic acids and flavonoids, sugars, proteins, amino acids, carotenes, organic acids, and water-soluble vitamins (vitamin B1, B2, B3, B9, B12, and vitamin C) [33][42].
Nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent antioxidant gene expression is noticeably reduced in COVID-19 patients. Nrf2 stimulators may inhibit the replication of SARS-CoV-2 as well as related inflammatory gene expression, as demonstrated for honey [12][43]. As stated above, inflammatory processes are also a hallmark of SARS-CoV-2 infection. The inflammatory markers mitogen-activated protein kinase (MAPK) and the nuclear factor kappa B (NF-κB) induce the production of several other inflammatory factors, such as enzymes, cytokines, proteins, and cyclooxygenase-2 (COX-2), lipoxygenase 2 (LOX-2), CRP, interleukins (IL-1β, IL-6, and IL-10), tumor necrosis factor α (TNF-α), granulocyte–macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), macrophage inflammatory protein (MIP1), MIP1A, MIP1B, platelet-derived growth factor (PDGF), and interferon-inducible protein 10 (IP-10) [31][35][41]. Recent in vitro and in vivo studies have demonstrated the anti-inflammatory mechanisms of honey. Hussein et al. [33] stated that honey decreases carrageenan-induced rat paw inflammation by attenuating NF-κB translocation to the nucleus, and by inhibition of the NF-κB degradation, with subsequent decrease of pro-inflammatory mediators COX-2 and TNF-α. Moreover, the same researchers reported that honey also inhibited the production of the proinflammatory mediators nitric oxide (NO), prostaglandin E2 (PGE(2)), TNF-α, and IL-6, using the same animal model [24].
The attachment of SARS-CoV-2 to the cell surface occurs via interaction with the ACE-2 receptor, which is present in several cell surfaces, including lungs, heart, kidney, and arteries. Upon virus entrance, viral particles are recognizable by pattern recognition receptors (e.g., TLR3, TLR4, and TLR7). Inside human cells, the immune system is activated, including macrophages, natural killer cells, CD4+ and CD8+ T-cells, B-cells, neutrophils, and dendritic cells, aimed at the destruction of SARS-CoV-2 [33]. It has been reported that honey stimulates B-lymphocytes and T-lymphocytes in cell culture to multiply and activate neutrophils, which induce cytokine production, such as, IL-1, IL-6, and TNF-α and apalbumin 1 (AP-1) [44]. A variety of honeys have been associated with the increase of immune responses mediators [45][46]. Tonks et al. [47] discovered a 5.8 kDA component of Manuka honey that stimulates the production of TNF-α in macrophages via TLR4, such as blockade suppress honey-mediated immunomodulatory effects.
Besides all these phenomena, honey active phagocytosis and autophagy for pathogen clearance. Phagocytes are the first line of defense of the innate immune system, it being demonstrated that honey provides a supply of glucose essential for the “respiratory burst” of phagocytes (such as, polymorphonuclear neutrophils (PMNs) and peripheral blood mononuclear cells (MNCs)) [48]. On the other hand, autophagy is a highly conserved catabolic process that allows the cell to remove long-lived proteins, lipid, unwanted or damaged cells, as well as impurities, helping to maintain healthier cells. Different researchers have reported that flavonoids, phenolic acids and MGO present in honey induce cell death by autophagy and by inhibition of the mTOR signaling pathway [49][50].
2.3. Cure and/or Improve Comorbid Conditions
Hyperglycemia has been found to be one of the causative risks factors of death in SARS-CoV-2 patients. Therefore, the hypoglycemic effect of honey previously reported could be of huge importance for this condition [51][52]. Meo et al. [52] stated that honey could decrease fasting serum glucose as well as triglycerides and low-density lipoproteins (LDLs), increasing high-density lipoproteins (HDLs), the fasting C-peptide level and the 2-h postprandial C-level.
Besides hyperglycemia, myocarditis also contributes towards the final severe outcomes observed in SARS-CoV-2 patients [53]. Impaired lipid metabolism can lead to the elevation of total cholesterol, LDL, and triglycerides (TGs). Excessive ROS can attack the elevated LDL to gradually form atherosclerotic plaques that can block blood supply to the myocardium, inducing a hypoxic state that ultimately leads to the myocardial tissue necrosis [54].
Several in vitro, in vivo, and clinical trial studies have revealed positive honey effects against heart problems by improving the plasma lipid profile (i.e., reduced the level of very low-density lipoprotein (VLDL), LDL, TG, cardiovascular risk predictive index (CVPI), plasma cholesterol, TC, and HDL) [55], suppressing oxidation, attenuating the elevation of cardiac damage markers (e.g., creatine kinase (CK–MB) and cardiac troponin I) as well as aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alanine aminotransferase (ALT) [56]. Increasing activities of antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase/reductase [57], and LDL resistance to oxidation [58], have been observed.
This entry is adapted from the peer-reviewed paper 10.3390/app12157800
References
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118.
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127.
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. 2017, 16, 1072–1100.
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313.
- Saranraj, P.; Sivasakthi, S.; Feliciano, G.D. Pharmacology of honey: A review. Adv. Biol. Res. 2016, 10, 271–289.
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. 2012, 9, 61.
- Tomás, A.; Falcão, S.I.; Russo-Almeida, P.; Vilas-Boas, M. Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. J. Apic. Res. 2017, 56, 219–230.
- Codex Alimentarius. Standard for Honey, CXS 12-19811 Adopted in 1981. Revised in 1987, 2001. Amended in 2019. Available online: https://www.fao.org (accessed on 28 June 2022).
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562.
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of markers of botanical origin and other compounds extracted from unifloral honeys. J. Agric. Food Chem. 2013, 61, 1747–1755.
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on manuka honey. Foods 2014, 3, 420–432.
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating nrf2, nf-κb and p38 mapk. Nutr. Metab. 2019, 16, 15.
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069.
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (COVID-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232.
- Abedi, F.; Ghasemi, S.; Farkhondeh, T.; Azimi-Nezhad, M.; Shakibaei, M.; Samarghandian, S. Possible potential effects of honey and its main components against COVID-19 infection. Dose Response 2021, 19, 1559325820982423.
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846.
- Shahzad, A.; Cohrs, R.J. In vitro antiviral activity of honey against varicella zoster virus (vzv): A translational medicine study for potential remedy for shingles. Transl. Biomed. 2012, 3, 2.
- Zeina, B.; Othman, O.; Al-Assad, S. Effect of honey versus thyme on rubella virus survival in vitro. J. Altern. Complement. Med. 1996, 2, 345–348.
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza viral effects of honey in vitro: Potent high activity of manuka honey. Arch. Med. Res. 2014, 45, 359–365.
- Abdel-Naby Awad, O.G.; Hamad, A.H. Honey can help in herpes simplex gingivostomatitis in children: Prospective randomized double blind placebo controlled clinical trial. Am. J. Otolaryngol. 2018, 39, 759–763.
- Abuelgasim, H.; Albury, C.; Lee, J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: A systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 57–64.
- Obossou, E.K.; Shikamoto, Y.; Hoshino, Y.; Kohno, H.; Ishibasi, Y.; Kozasa, T.; Taguchi, M.; Sakakibara, I.; Tonooka, K.; Shinozuka, T.; et al. Effect of manuka honey on human immunodeficiency virus type 1 reverse transcriptase activity. Nat. Prod. Res. 2021, 36, 1552–1557.
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Altern. Med. 2021, 21, 30.
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE(2), TNF-α, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 109636.
- Rossi, M.; Marrazzo, P. The potential of honeybee products for biomaterial applications. Biomimetics 2021, 6, 6.
- Elmahallawy, E.K.; Mohamed, Y.; Abdo, W.; El-Gohary, F.A.; Ahmed Awad Ali, S.; Yanai, T. New insights into potential benefits of bioactive compounds of bee products on COVID-19: A review and assessment of recent research. Front. Mol. Biosci. 2020, 7, 618318.
- Tang, S.-W.; Ducroux, A.; Jeang, K.-T.; Neuveut, C. Impact of cellular autophagy on viruses: Insights from hepatitis b virus and human retroviruses. J. Biomed. Sci. 2012, 19, 92.
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M. Portuguese honeys from different geographical and botanical origins: A 4-year stability study regarding quality parameters and antioxidant activity. Molecules 2017, 22, 1338.
- Curuțiu, C.; Dițu, L.M.; Grumezescu, A.M.; Holban, A.M. Polyphenols of honeybee origin with applications in dental medicine. Antibiotics 2020, 9, 856.
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160.
- Al-Waili, N.S. Topical honey application vs. Acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, Mt94-8.
- Charyasriwong, S.; Haruyama, T.; Kobayashi, N. In vitro evaluation of the antiviral activity of methylglyoxal against influenza b virus infection. Drug Discov. Ther. 2016, 10, 201–210.
- Hossain, K.S.; Hossain, M.G.; Moni, A.; Rahman, M.M.; Rahman, U.H.; Alam, M.; Kundu, S.; Rahman, M.M.; Hannan, M.A.; Uddin, M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon 2020, 6, e05798.
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J. Agric. Food Chem. 2019, 67, 688–698.
- Corrêa, T.A.F.; Rogero, M.M. Polyphenols regulating micrornas and inflammation biomarkers in obesity. Nutrition 2019, 59, 150–157.
- Güler, H.İ.; Ay Şal, F.; Can, Z.; Kara, Y.; Yildiz, O.; Beldüz, A.O.; Çanakçi, S.; Kolayli, S. Targeting cov-2 spike rbd and ace-2 interaction with flavonoids of anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics. Turk. J. Biol. 2021, 45, 530–548.
- Shaldam, M.A.; Yahya, G.; Mohamed, N.H.; Abdel-Daim, M.M.; Al Naggar, Y. In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes. Environ. Sci. Pollut. Res. Int. 2021, 28, 40507–40514.
- Hussain, F.; Jahan, N.; Rahman, K.-u.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ace) inhibition potential. Oxid. Med. Cell. Longev. 2018, 2018, 4643736.
- Kalediene, L.; Baz, M.; Liubaviciute, A.; Biziuleviciene, G.; Grabauskyte, I.; Bieliauskiene, R.; Jovaisas, P.; Jurjonas, N. Antiviral effect of honey extract camelyn against SARS-CoV-2. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 290–297.
- Al Naggar, Y.; Giesy, J.P.; Abdel-Daim, M.M.; Javed Ansari, M.; Al-Kahtani, S.N.; Yahya, G. Fighting against the second wave of COVID-19: Can honeybee products help protect against the pandemic? Saudi J. Biol. Sci. 2021, 28, 1519–1527.
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with covid-19. Eur. Heart J. 2021, 42, 2270–2279.
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013, 313798.
- Ogawa, T.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Watanabe, R.; Fujisawa, Y.; Fujimoto, M. Honey and chamomile activate keratinocyte antioxidative responses via the keap1/nrf2 system. Clin. Cosmet. Investig. Dermatol. 2020, 13, 657–660.
- Manyi-loh, C.E.; Clarke, A.M.; Ndip, R.N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852.
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Alternat. Med. 2011, 2011, 908743.
- Bíliková, K.; Šimúth, J. New criterion for evaluation of honey: Quantification of royal jelly protein apalbumin 1 in honey by Elisa. J. Agric. Food Chem. 2010, 58, 8776–8781.
- Tonks, A.J.; Dudley, E.; Porter, N.G.; Parton, J.; Brazier, J.; Smith, E.L.; Tonks, A. A 5.8-kda component of manuka honey stimulates immune cells via tlr4. J. Leukoc. Biol. 2007, 82, 1147–1155.
- Mesaik, M.A.; Azim, M.K.; Mohiuddin, S. Honey modulates oxidative burst of professional phagocytes. Phytother. Res. 2008, 22, 1404–1408.
- Klappan, A.K.; Hones, S.; Mylonas, I.; Brüning, A. Proteasome inhibition by quercetin triggers macroautophagy and blocks mtor activity. Histochem. Cell Biol. 2012, 137, 25–36.
- Lee, J.H.; Parveen, A.; Do, M.H.; Kang, M.C.; Yumnam, S.; Kim, S.Y. Molecular mechanisms of methylglyoxal-induced aortic endothelial dysfunction in human vascular endothelial cells. Cell Death Dis. 2020, 11, 403.
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Fructose might contribute to the hypoglycemic effect of honey. Molecules 2012, 17, 1900–1915.
- Meo, S.A.; Ansari, M.J.; Sattar, K.; Chaudhary, H.U.; Hajjar, W.; Alasiri, S. Honey and diabetes mellitus: Obstacles and challenges—Road to be repaired. Saudi J. Biol. Sci. 2017, 24, 1030–1033.
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from wuhan, china. Int. Care Med. 2020, 46, 846–848.
- Bt Hj Idrus, R.; Sainik, N.Q.A.V.; Nordin, A.; Saim, A.B.; Sulaiman, N. Cardioprotective effects of honey and its constituent: An evidence-based review of laboratory studies and clinical trials. Int. J. Environ. Res. Public Health 2020, 17, 3613.
- Alagwu, E.A.; Okwara, J.E.; Nneli, R.O.; Osim, E.E. Effect of honey intake on serum cholesterol, triglycerides and lipoprotein levels in albino rats and potential benefits on risks of coronary heart disease. Niger J. Physiol. Sci. 2011, 26, 161–165.
- Afroz, R.; Tanvir, E.M.; Karim, N.; Hossain, M.S.; Alam, N.; Gan, S.H.; Khalil, M.I. Sundarban honey confers protection against isoproterenol-induced myocardial infarction in wistar rats. BioMed. Res. Int. 2016, 2016, 6437641.
- Rakha, M.K.; Nabil, Z.I.; Hussein, A.A. Cardioactive and vasoactive effects of natural wild honey against cardiac malperformance induced by hyperadrenergic activity. J. Med. Food 2008, 11, 91–98.
- Busserolles, J.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J. Nutr. 2002, 132, 3379–3382.
This entry is offline, you can click here to edit this entry!
