Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Allicin, a sulfur compound naturally derived from garlic, has shown beneficial effects on several cardiovascular risk factors through the modulation of cellular mechanisms and signaling pathways. Garlic is especially rich in sulfur-containing compounds; thus, many of these compounds can be responsible for its therapeutic effects. Recent studies have shown that allicin, a garlic-derived sulfur compound, has beneficial effects on different cell types that could be useful for the management of CVD or its risk factors.
- allicin
- cardiovascular disease
- risk factors
- oxidative stress
- hypertension
- dyslipidemia
- endothelial dysfunction
- inflammation
- myocardial infarction
- apoptosis
1. Allicin
1.1. Garlic as a Natural Source of Allicin
Garlic is useful in the treatment of CVD, mainly due to its anti-inflammatory, anti-hypertensive, anti-platelet, and anti-diabetic effects [1][2][3][4][5]. However, recent studies suggest that the biological activities in garlic can be attributed to allicin, the compound formed in high proportion when a garlic clove is cut, macerated, or crushed. Therefore, it has been hypothesized that allicin is the main compound responsible for the beneficial effects of garlic consumption.
Raw garlic water content is approximately 50%, the rest consists of carbohydrates, lipids, proteins, fiber, vitamins, free amino acids, and minerals, and it is especially rich in sulfur compounds (Table 1) [2][3][4][6].
Garlic is odorless until the alliin compound [(+)-S-(2-propenyl)-L-cysteine sulfoxide] and the alliinase enzyme react. These components in intact garlic bulbs are stored in vesicles or vacuoles independently until mechanical stimuli such as cutting or maceration break it, thus, alliin and alliinase are released and the catalysis for allicin formation takes place (Figure 1A) [6]. There are other sulfur compounds in raw garlic, but the proportion of these is lower compared to the alliin.
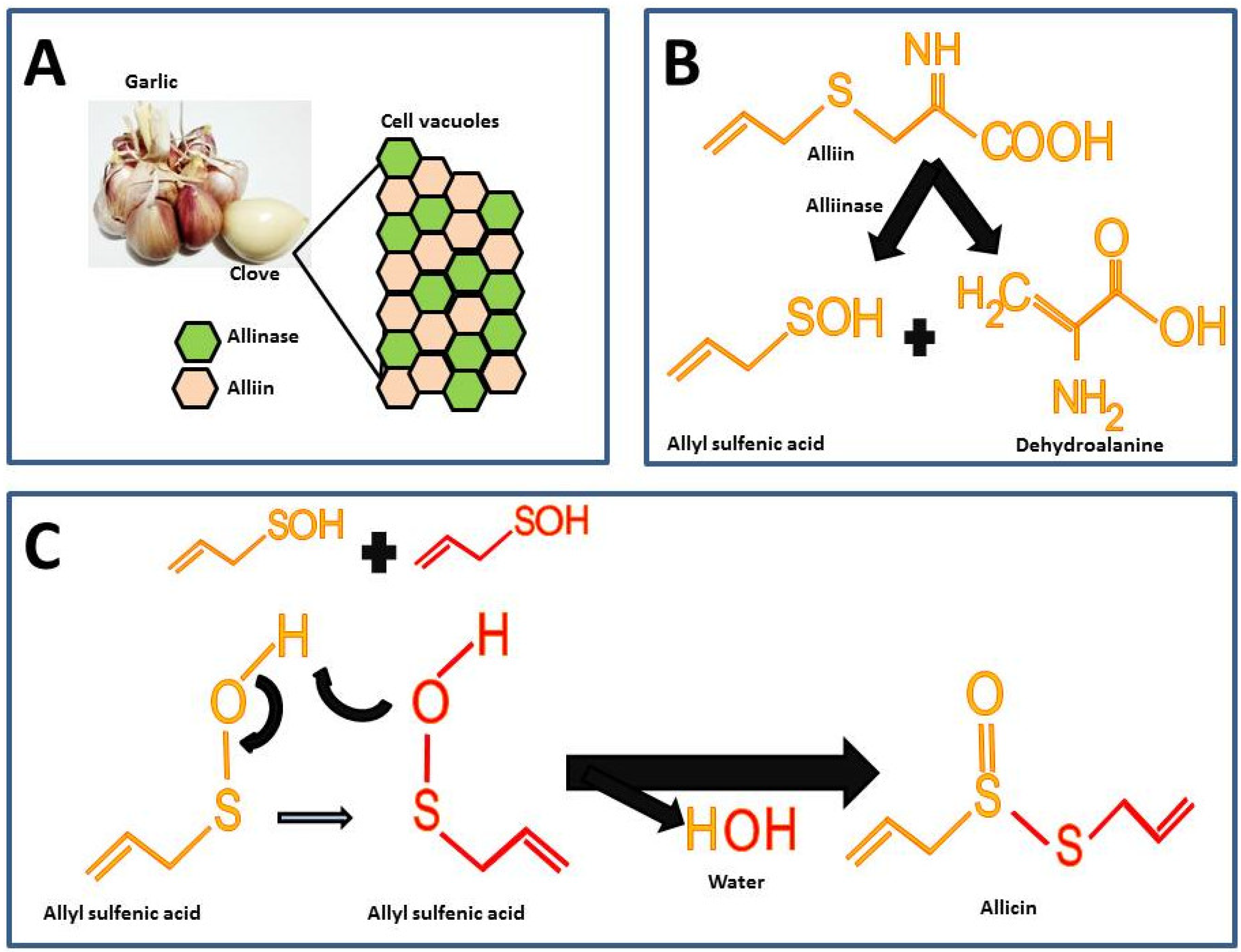
Figure 1. Chemical reactions for allicin synthesis in crushed garlic cloves and substrate formation for chemical synthesis: (A) Compartmentalization of alliin and alliinase in the intact garlic bulb; (B) First reaction in allicin formation; (C) Second reaction for the allicin formation.
The highest percentage of sulfur compounds in garlic is represented by γ-L-glutamyl-S-(2-propenyl)-L-cysteine (GSAC) an alliin precursor, which is transformed into alliin the substrate in the allicin synthesis reaction [6]. For the synthesis of allicin, alliin is hydrolyzed by the enzyme alliinase into allyl sulfenic acid and dehydroalanine (Figure 1B) [7]. Allicin is formed by the reaction from two allyl sulfenic acid molecules, resulting in allicin and one water molecule as a final product (Figure 1C).
The allicin synthesized represents 60 to 80% of all sulfur compounds (Table 1) and it gives the characteristic odor and hence is considered the active compound in garlic [4]. Furthermore, in spite of the fact that alliin and allicin have similar chemical residues, alliin does not show the biological activity of allicin [8][9]. In fact, a recent study assessed the effect of alliin on experimental obesity resulting in modest beneficial effects [9]. The latter supports the hypothesis that allicin is the active compound responsible for the biological activities in garlic.
Table 1. Composition of raw garlic (Allium sativum).
| Substance or Compound | Percent in 100 g Dry Weight | |
|---|---|---|
| Water | 50% | |
| Carbohydrates | 30% | |
| Proteins | 10% | |
| Alliinase | 10 mg/gr | |
| Free amino acid | 1.0% | |
| Lipids | 3.5% | |
| Fiber | 1.0% | |
| Kilocalories | 149 Kcal | |
| Vitamins | ||
| B1 | 0.16 mg | |
| B2 | 0.02 mg | |
| B6 | 0.32 mg | |
| Nicotinic Acid | 0.12 mg | |
| Ascorbic Acid | 14 mg | |
| Minerals | ||
| Potassium | 446 mg | |
| Phosphorous | 134 mg | |
| Sodium | 19 mg | |
| Calcium | 17 mg | |
| Iron | 1.2 mg | |
| Magnesium | 24.1 mg | |
| Zinc | 1.1 mg | |
| Iodine | 4.7 µg | |
| Selenium | 2 µg | |
| Sulfur compounds | 3.5% | |
| γ-glutamyl peptides: 80–85% | ||
| γ-L-glutamyl-S-(2-propenyl)-L-cysteine (GSAC) | 40–60% | |
| γ-L-glutamyl-S-(trans-1-propenil)-L-cysteine (GSPC) | 10–18% | |
| γ-L-glutamyl-S-methyl-L-cysteine (GSMC) | 10–18% | |
| Sulfoxides produced by the allinase action: | ||
| (+)-S-(2-propenyl)-L-cysteine sulfoxide (alliin) | 60–80% | |
| (+)-S-(trans-1-propenil)-L-cysteine sulfoxide (isoalline) | ||
| (+)-S-methyl-L-cysteine sulfoxide (methiine) | ||
| (1S, 3R, 5S) -5-methyl-1, 4-thiazan-3-carboxylic acid 1-oxide (cycloaliine). |
1.2. Synthetic Allicin
Allicin can also be obtained by chemical synthesis by a reverse process from the garlic decomposition cascade of natural synthesis. Briefly, diallyl disulfide dissolved in acetic acid is mixed with hydrogen peroxide to form allyl sulfenic acid, which, as described above, is the substrate for spontaneous condensation producing allicin (Figure 1C) that is further extracted from water with a polar organic solvent as dichloromethane. Through this synthetic procedure, allicin can be obtained with a high purity ranging from 90–92% [13]. Due to its hydrophobic nature, allicin can easily diffuse the phospholipid bilayer in cell membranes. Furthermore, it is known that allicin rapidly reacts with the free thiol or sulfhydryl groups of amino acids such as cysteine and the proteins on the cell membrane, as well as with other proteins in the cell compartments [14][15]. These characteristics could be important for the induction of the biological activities attributed to allicin.
2. Effects of Allicin on Cardiovascular Risk Factors
The treatment for CVD is mainly focused on controlling risk factors [16][17] and commonly includes pharmacological approaches and changes in lifestyle [18][19]. Until now, this type of intervention has been unsuccessful, because two or more drugs are required for optimal control of risk factors and usually have undesirable secondary effects. For this reason, the search for therapeutic options that contribute to preventing or delaying the progression of CVD continues.
CVD has a multifactorial origin, which makes its treatment difficult, thus the objective of the present study was to collect the available information on allicin and its beneficial effects on cardiovascular risk factors. In summary, data from experimental and clinical studies show that allicin decreases hypertension, inflammation, platelet aggregation, hyperglycemia, fibrosis, oxidative stress, and apoptosis and improves lipid profile, arrhythmias, and endothelial and cardiac function. For those reasons, and its low toxicity [5][6][20][21] allicin may be a reliable option for the treatment of the different clinic manifestations of CVD and its risk factors.
Allicin is an oily liquid, bright yellow in color, unstable, and with a short half-life [7]. However, due to its hydrophobic nature, it can be readily absorbed through cell membranes without inducing any damage to the phospholipid bilayer [22]. In this context, it has been reported that allicin is able to cross the erythrocyte cell membrane [15]. Possibly in this way, allicin can be distributed to various cell types, organs, and systems, including the CS to exert its beneficial effects. On a molecular level, the primary effect of allicin seems to be as an antioxidant and the multiple downstream effects described could be due to an indirect effect, since it can act as a bifunctional antioxidant. Due to its structure, allicin can act as a direct antioxidant by reacting directly with free radicals and ROS, acting as a substrate for glutathione synthesis, reacting with glutathione to produce S-Allyl-mercaptan glutathione, with L-cysteine to produce S-Allyl-mercaptan cysteine or other molecules that also can act as antioxidants [23][24]. On the other hand, allicin as an indirect antioxidant modulates the expression of cytoprotective genes. It is known that allicin reacts rapidly with the free thiol or sulfhydryl groups of the proteins in the cell membrane or in the different cell compartments [14][15]. It is likely that through this mechanism, allicin modifies proteins, second messengers, or transcription factors implicated in the regulation of gene expression. The regulation in the expression of antioxidant enzymes related to the Nrf2/Keap1 signaling pathway is possibly one of the well-known mechanisms through which allicin exerts antioxidant effects and has been considered an indirect mechanism [25][26][27][28]. Furthermore, it is tempting to hypothesize that the anti-inflammatory mechanisms of allicin related to the NF-κB/IκB-dependent pathway could be associated with activation or inhibition via the modification of sulfhydryl groups. However, further studies are required to confirm or discard the presence of sulfhydryl groups derivatives by allicin as the responsible mechanism involved in the regulation of cytoprotective gene expression. The antihypertensive effects of allicin may be exerted through the improvement of endothelial function and the modulation of proteins and substances associated with vasoactive responses (eNOS, angiotensin II receptors type 1 and type 2 (AT1R and AT2R), ET-1, cGMP, angiotensin, NO, and H2S). Through in vitro assays, it was demonstrated that allicin decreased the vascular reactivity to angiotensin II, and AT1R overexpression in the heart [26]. Another antihypertensive effect of allicin was demonstrated by its effects on the stimulation of the AT2R expression in renal tissue in CKD [29]. Moreover, the antioxidant effects of allicin could contribute to increasing the half-life of the vasoactive metabolites such as NO, and therefore to the maintenance of the vasodilatory actions. Additionally, the cardioprotective effects of allicin have been evidenced in the prevention of apoptosis of ischemic areas, as well as the induction of angiogenesis, which could be useful for the treatment of myocardial infarction, one of the first death causes worldwide [30][31][32][33][34][35]. The antiapoptotic and angiogenic activities of allicin may be useful to restore blood flow inducing recovery of injured areas and the effects on the modulation of expression and function of ion channels open the possibility of the use of allicin as an antiarrhythmic agent and pro-angiogenic.
A possible disadvantage of allicin is its poor stability and half-life. Thus, to improve the stability and half-life of allicin, a group of researchers conjugated allicin and captopril producing S-allylmercaptocaptopril (CPSSA). This compound was more stable and showed antihypertensive, lipid-lowering, and homocysteine-reducing effects, leading to the proposal of the use of this new conjugated molecule as a therapeutic option for the treatment of CVD [36][37]. Another study using an allicin-fenofibrate combination reported synergistic effects on the regulation of blood lipids and the improvement of vascular endothelial function [38]. For improving the stability and half-life of allicin, and for improving delivery and releasing, investigations have reported delivery systems including emulsion gels, nano emulsions, liposomes, microcapsules, and enteric-coated tablets [6][39][40][41].
Regarding the dose or concentration of allicin needed to obtain cardioprotective effects, allicin has demonstrated beneficial effects at low doses such as 8 mg/kg/day in experimental models [42]. The clinical studies have been carried out in patients with cardiovascular disease and healthy volunteers aged 20 years or older and the doses used a range from 10 to 48 mg/kg/day. No side effects, included bad smell, were reported in short-term or long-term studies [5][6][20][21][43]. However, healthy volunteers reported abdominal discomfort and stomachache subsequent to the administration of 48 mg of allicin once a day during dinner for 1 week on an empty stomach [44]. The intake of allicin from some garlic presentations has caused bad body odor and breath, possibly because quantities greater than one gram of garlic are necessary to obtain allicin in >0.6 mg [6]. No study has used allicin in pediatric or children’s populations. A report assessed garlic extract therapy at 300 mg, 3 times a day/8 weeks, and no significant effect was observed on CVD risk factors in pediatric patients (8–18 years) with familial hyperlipidemia [45]. However, in adolescents, the garlic dry extract is recommended as a single dose (100–200 mg) once or twice a day, and the use in children under 12 years of age is not recommended [11]. Further studies are needed and will provide information about the safety, care, efficacy, and recommendations for the use of allicin in sensitive populations such as infants and children. Therefore, controlled clinical studies and trials with younger and pediatric patients are recommended to provide information about side effects or further data to support the use of allicin as a pharmacological option for the treatment of risk factors, pathogenic mechanisms, and clinical manifestations of CVD.
Garlic is a spice that has been attributed to beneficial effects on CV risk factors even since ancient times [10], but, so far, it is not used as a therapeutic option mainly because there are no standardized data on dosage, there are no specified garlic-derived compounds, or controlled studies on pharmacokinetics and pharmacodynamics, that allows replicating the beneficial effects. Recently, research has focused on studying the purified garlic compounds, showing important effects on various diseases [46][47]. Garlic has several sulfur compounds and depending on the species, maturation, processing method, or presentation, the quantity of one type of compound or another predominates [12]. In this context, allicin a garlic-derived compound has been isolated, purified or synthesized, quantified, characterized, and used in different dosages for the treatment of several cardiovascular diseases [6][22]. Despite the possible disadvantage of its poor stability [22] and half-life, the scientific literature provides convincing data that allicin alone or combined with anti-hypertensive [36][37][48][49] or anti-dyslipidemia drugs [38] may provide cardioprotective effects. In this sense, the use of delivery systems may be useful to increase absorption, bioavailability and efficacy, thus the biological activities and health benefits [6]. Allicin has shown beneficial effects on several metabolic alterations, risk factors, and cell injury. These effects have been observed in various organs, systems, and cell types (i.e., heart, endothelium, liver, and kidney). Therefore, this work provides a landscape of the scientific literature focusing on the beneficial effects of allicin on risk factors of CVD, as well as the cellular and molecular mechanisms involved.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169082
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559.
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century–a review. Asian Pac. J. Trop. Dis. 2015, 5, 271–278.
- Bose, S.; Laha, B.; Banerjee, S. Anti-inflammatory activity of isolated allicin from garlic with post-acoustic waves and microwave radiation. J. Adv. Pharm. Educ. Res. 2013, 3, 512–515.
- Villamiel, M.; Corzo-Martinez, M.; Soria, A.C. A comprehensive survey of Garlic functionality. Garlic Consum. Health 2010, 5, 642–649.
- Ashraf, R.; Aamir, K.; Shaikh, A.R.; Ahmed, T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J. Ayub Med Coll. Abbottabad JAMC 2005, 17, 60–64.
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812.
- Ilic, D.; Nikolic, V.; Nikolic, L.; Stankovic, M.; Stanojevic, L.; Cakic, M. Allicin and related compounds: Biosynthesis, synthesis and pharmacological activity. Facta Univ.-Ser. Phys. Chem. Technol. 2011, 9, 9–20.
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 3527.
- Sánchez-Sánchez, M.A.; Zepeda-Morales, A.S.M.; Carrera-Quintanar, L.; Viveros-Paredes, J.M.; Franco-Arroyo, N.N.; Godínez-Rubí, M.; Ortuño-Sahagun, D.; López-Roa, R.I. Alliin, an Allium sativum Nutraceutical, ReducesMetaflammation Markers in DIO Mice. Nutrients 2020, 12, 624.
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14.
- Redondo, G.L.M.; Gutiérrez, A.L.; Barrantes, J.B.; Navarro, M.P.; Monge, M.C.O.; Vargas, M.J.C. Aspectos generales del Allium sativumuna revisión. Ars Pharm. 2021, 62, 471–481.
- Cardelle, A.; Soria, A.; Corzo, N.; Villamiel, M. A Comprehensive Survey of Garlic Functionality; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 1–60.
- Argüello-García, R.; Medina-Campos, O.N.; Pérez-Hernández, N.; Pedraza-Chaverrí, J.; Ortega-Pierres, G. Hypochlorous Acid Scavenging Activities of Thioallyl Compounds from Garlic. J. Agric. Food Chem. 2010, 58, 11226–11233.
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of Allicin Determined by Chemical and Biological Assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883.
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta-Biomembr. 2000, 1463, 20–30.
- Corrigendum to: European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 4507.
- Jorge-Galarza, E.; Martínez-Sánchez, F.D.; Javier-Montiel, C.I.; Msc, A.X.M.; Posadas-Romero, C.; Rd, M.C.G.; Osorio-Alonso, H.; Msc, A.S.A.; Juárez-Rojas, J.G. Control of blood pressure levels in patients with premature coronary artery disease: Results from the Genetics of Atherosclerotic Disease study. J. Clin. Hypertens. 2020, 22, 1253–1262.
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M.; et al. Challenges and Opportunities for the Prevention and Treatment of Cardiovascular Disease Among Young Adults: Report From a National Heart, Lung, and Blood Institute Working Group. J. Am. Heart Assoc. 2020, 9, e016115.
- Medina-Urrutia, A.X.; Martínez-Sánchez, F.D.; Posadas-Romero, C.; Jorge-Galarza, E.; Martínez-Alvarado, M.D.R.; González-Salazar, M.D.C.; Osorio-Alonso, H.; Juárez-Rojas, J.G. Metabolic control achievement in a population with premature coronary artery disease: Results of the genetics of atherosclerotic disease study. Ther. Adv. Endocrinol. Metab. 2020, 11, 1–10.
- Soleimani, D.; Moosavian, S.P.; Zolfaghari, H.; Paknahad, Z. Effect of garlic powder supplementation on blood pressure and hs-C-reactive protein among nonalcoholic fatty liver disease patients: A randomized, double-blind, placebo-controlled trial. Food Sci. Nutr. 2021, 9, 3556–3562.
- McMahon, F.G.; Vargas, R. Can garlic lower blood pressure? A pilot study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1993, 13, 406–407.
- Chan, J.Y.-Y.; Yuen, A.C.-Y.; Chan, R.Y.-K.; Chan, S.-W. A Review of the Cardiovascular Benefits and Antioxidant Properties of Allicin. Phytother. Res. 2013, 27, 637–646.
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.-X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844.
- Pedraza-Chaverrí, J.; Barrera, D.; Maldonado, P.D.; Chirino, Y.I.; Macías-Ruvalcaba, N.A.; Medina-Campos, O.N.; Castro, L.; Salcedo, M.I.; Hernández-Pando, R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin. Pharmacol. 2004, 4, 5.
- Li, X.-H.; Li, C.-Y.; Xiang, Z.-G.; Hu, J.-J.; Lu, J.-M.; Tian, R.-B.; Jia, W. Allicin Ameliorates Cardiac Hypertrophy and Fibrosis through Enhancing of Nrf2 Antioxidant Signaling Pathways. Cardiovasc. Drugs Ther. 2012, 26, 457–465.
- García-Trejo, E.M.A.; Arellano-Buendía, A.S.; Argüello-García, R.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Arellano-Mendoza, M.G.; Castillo-Hernández, M.C.; Guevara-Balcázar, G.; Tapia, E.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Hypertension and Cardiac Function in Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2016, 2016, 3850402.
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018, 19, 3107.
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur. J. Nutr. 2008, 48, 67–74.
- Trejo, E.M.G.; Buendía, A.S.A.; Reyes, O.S.; Arroyo, F.E.G.; Garcia, F.; Mendoza, M.L.L.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. The Beneficial Effects of Allicin in Chronic Kidney Disease Are Comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401.
- Ma, L.-N.; Li, L.-D.; Li, S.-C.; Hao, X.-M.; Zhang, J.-Y.; He, P.; Li, Y.-K. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin. J. Integr. Med. 2016, 23, 589–597.
- Gao, T.; Yang, P.; Fu, D.; Liu, M.; Deng, X.; Shao, M.; Liao, J.; Jiang, H.; Li, X. The protective effect of allicin on myocardial ischemia-reperfusion by inhibition of Ca2+ overload-induced cardiomyocyte apoptosis via the PI3K/GRK2/PLC-γ/IP3R signaling pathway. Aging 2021, 13, 19643–19656.
- Xu, W.; Li, X.-P.; Li, E.-Z.; Liu, Y.-F.; Zhao, J.; Wei, L.-N.; Ma, L. Protective Effects of Allicin on ISO-Induced Rat Model of Myocardial Infarction via JNK Signaling Pathway. Pharmacology 2020, 105, 505–513.
- Liu, M.; Yang, P.; Fu, D.; Gao, T.; Deng, X.; Shao, M.; Liao, J.; Jiang, H.; Li, X. Allicin protects against myocardial I/R by accelerating angiogenesis via the miR-19a-3p/PI3K/AKT axis. Aging 2021, 13, 22843–22855.
- Miron, T.; Rabinkov, A.; Peleg, E.; Rosenthal, T.; Mirelman, D.; Wilchek, M. Allylmercaptocaptopril: A new antihypertensive drug. Am. J. Hypertens. 2004, 17, 71–73.
- Oron-Herman, M.; Rosenthal, T.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Sela, B.-A. The effects of S-allylmercaptocaptopril, the synthetic product of allicin and captopril, on cardiovascular risk factors associated with the metabolic syndrome. Atherosclerosis 2005, 183, 238–243.
- Li, W.; Wang, D.; Song, G.; Zuo, C.; Qiao, X.; Qin, S. The effect of combination therapy of allicin and fenofibrate on high fat diet-induced vascular endothelium dysfunction and liver damage in rats. Lipids Health Dis. 2010, 9, 131.
- Lu, Q.; Lu, P.-M.; Piao, J.-H.; Xu, X.-L.; Chen, J.; Zhu, L.; Jiang, J.-G. Preparation and physicochemical characteristics of an allicin nanoliposome and its release behavior. LWT Food Sci. Technol. 2014, 57, 686–695.
- Ma, C.; Li, S.; Yin, Y.; Xu, W.; Xue, T.; Wang, Y.; Liu, X.; Liu, F. Preparation, characterization, formation mechanism and stability of allicin-loaded emulsion gel. LWT 2022, 161, 113389.
- Wang, Y.-F.; Shao, J.-J.; Wang, Z.-L.; Lu, Z.-X. Study of allicin microcapsules in β-cyclodextrin and porous starch mixture. Food Res. Int. 2012, 49, 641–647.
- Dubey, H.; Singh, A.; Patole, A.M.; Tenpe, C.R.; Ghule, B.V. Allicin, a SUR2 opener: Possible mechanism for the treatment of diabetic hypertension in rats. Rev. Bras. de Farm. 2012, 22, 1053–1059.
- Liu, D.; Wang, S.; Li, J.; Liang, E.; Yan, M.; Gao, W. Allicin improves carotid artery intima-media thickness in coronary artery disease patients with hyperhomocysteinemia. Exp. Ther. Med. 2017, 14, 1722–1726.
- Schiattarella, G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956.
- McCrindle, B.W.; Helden, E.; Conner, W.T. Garlic Extract Therapy in Children with Hypercholesterolemia. Arch. Pediatr. Adolesc. Med. 1998, 152, 1089–1094.
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619.
- Asdaq, S.M.B.; Yasmin, F.; Alsalman, A.J.; Al Mohaini, M.; Kamal, M.; Al Hawaj, M.A.; Alsalman, K.J.; Imran, M.; Sreeharsha, N. Obviation of dyslipidemia by garlic oil and its organosulfur compound, diallyl disulphide, in experimental animals. Saudi J. Biol. Sci. 2021, 29, 2520–2525.
- Elkayam, A.; Mirelman, D.; Peleg, E.; Wilchek, M.; Miron, T.; Rabinkov, A.; Sadetzki, S.; Rosenthal, T. Allicin Enalapril on Blood Pressure, Insulin and Triglycerides Levels in Fructose-Induced Hyperinsulinemic-Hyperlipidemic Hypertensive Rats. Am. J. Hyperten. 2000, 14, 377–381.
- Elkayam, A.; Mirelman, D.; Peleg, E.; Wilchek, M.; Miron, T.; Rabinkov, A.; Oron-Herman, M.; Rosenthal, T. The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am. J. Hypertens. 2003, 16, 1053–1056.
This entry is offline, you can click here to edit this entry!
