Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Graphene oxide (GO), an oxidized form of graphene, is regarded to be more superior to graphene with regards to application in drug delivery due to the presence of functional groups that could allow the binding of different compounds, especially water-insoluble drugs.
- graphene oxide
- infectious disease
- antibacterial
- multidrug-resistant
1. Introduction
Infectious diseases represent a continuous and major threat to the worldwide suspension, and recently, they have become the main cause of enormous morbidity and mortality in all parts of the world [1]. The number of infectious diseases that caused by the growing of drug-resistant bacteria is ascending time by time, and the increased number of hospitalized patients with immunodeficiency has resulted in an increase of severe and invasive infections [2]. Due to these problems, many antibacterial agents exist for use against a wide range of infectious diseases, which can either be synthetic, plant and animal in origin or chemically modified natural compounds [3]. However, these bacteria acknowledge a remarkable effectiveness to adapt, survive and evolve by developing resistance against antibacterial compounds, which leads to the global spread of resistant microorganisms towards antibiotics [4]. While antibiotics supposedly become effective means to control such infections, antibiotic overuse triggers the spread of resistant strains in the population. As a result, many strains of dangerous bacterial pathogens nowadays are resistant to antibiotics, with some strains combining even multiple resistances to different antibiotics [5]. In the current era of multidrug resistance, in which bacteria are gaining resistance to many antimicrobial agents, it is becoming very difficult for healthcare workers to treat patients, leading to severe morbidity and mortality [6]. With the current increase in prevalence of MDR bacteria, there will be no more efficient antibiotics by 2050 [7]. The process of drug discovery and clinical trials of new antibacterial drugs take a long time; hence, only a few new agents have recently been approved and are available for use [8]. The emergence and spread of bacteria resistant to multiple antibacterial agents (so-called “superbugs”), due to mutations in the pathogens and overuse of antimicrobials, should be considered as a cause that triggers serious concern [6]. Commonly, the bacteria can acquire extrachromosomal genetic elements that contain the gene encoding the virulence factor leading to antimicrobial resistance. This occurs via horizontal gene transfer. Some defence mechanisms (Figure 1) include the difference in bacterial cell wall compositions, production of degrading enzymes against antibiotics, action of efflux pumps and modification of antibiotic targets leading to tolerance and resistance of the therapy [7].
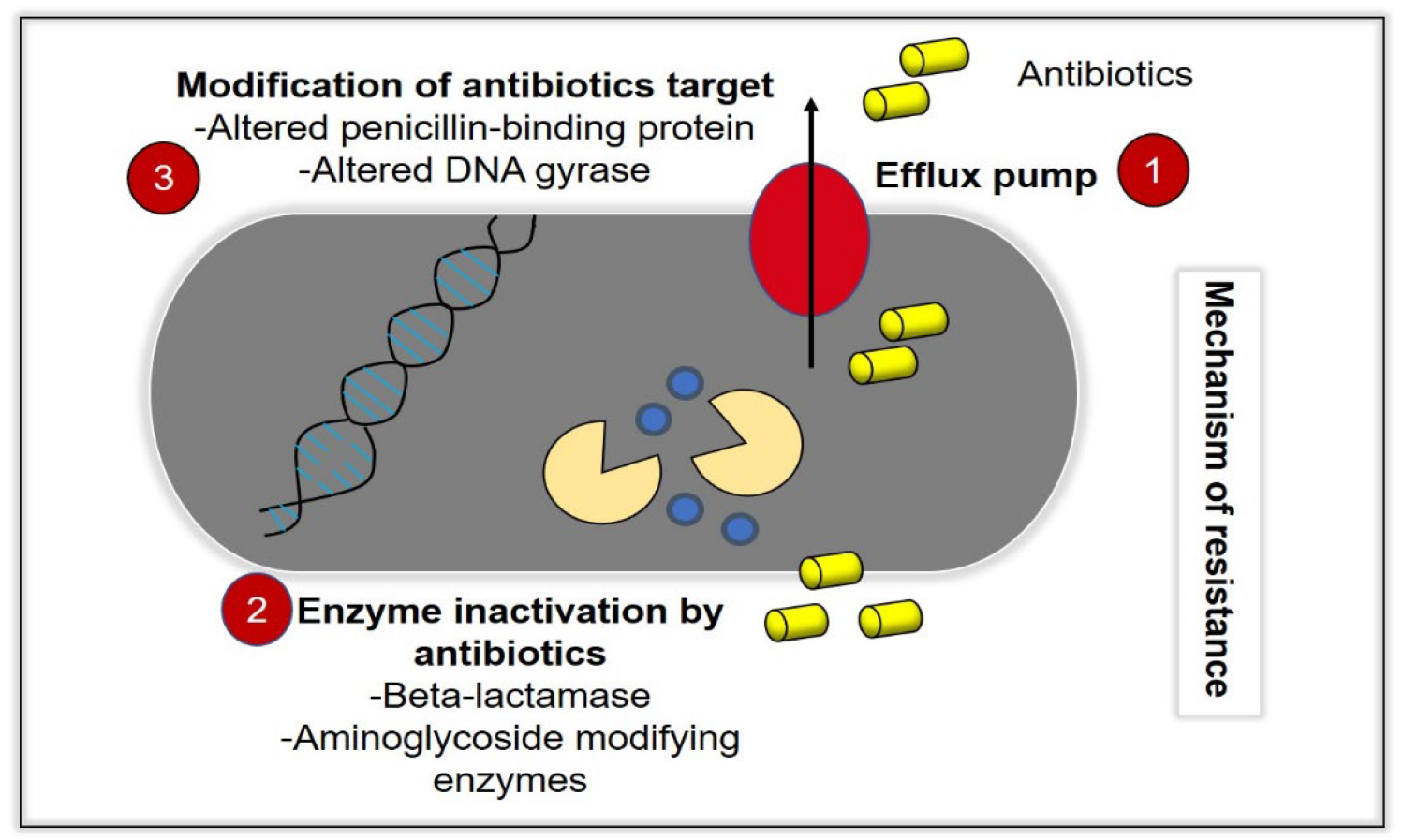

Figure 1. Bacteria acquire resistance toward antibacterial agents (antibiotics) through various mechanisms. (1) Efflux pump activity, in which the intracellular antibiotics are removed from the bacteria cells, which prevents the accumulation of antibiotics at its therapeutic concentrations, intracellularly. (2) Inactivation of enzymes, such as beta-lactamase, and (3) modification of antibiotics targets, such as alterations of the penicillin-binding protein (ABP) and DNA gyrase.
2. Graphene Oxide
A plethora of studies have demonstrated the potential future application of nanomaterials in various disciplines. Nanomaterials can be employed in modality treatments, including targeted drug delivery systems, as well as integration into nanoscale probes for bioimaging, attributed to its unique physiochemical properties, enhanced durability and versatility. Recent advancements in technology coupled with researchers exploring nanomaterial applications in the health industry, have led to new innovative and promising solutions to problems that conventional tools have yet to solve. However, nanomaterial research has also raised new concerns, especially regarding its biocompatibility, which have prompted studies to assess the potential toxicity of nanomaterials.
Graphene oxide (GO) has been chosen as the nanomaterial of interest in the present research due to its tremendous potential. Graphene oxide (GO), an oxidized form of graphene, is regarded to be more superior to graphene with regards to application in drug delivery due to the presence of functional groups that could allow the binding of different compounds, especially water-insoluble drugs. The highly oxidized structure of GO could also provide better biocompatibility and permeability in the aqueous environment common within the human body, allowing it to be more effective in the targeted drug delivery system [9]. GO possessed the highest antimicrobial activity rather than another graphene-derived material [10]. In recent years, there have been many studies demonstrating the antibacterial effects of GO on various types of bacteria, including Gram-positive and Gram-negative bacteria, as shown in the table below. The antibacterial activities shown in these studies employed different experimental methods and data presentation, such as the zone of inhibition, colony counting and methods to indicate and quantify viable and dead cells by means of metabolic activity and fluorescent DNA. These studies also used different concentrations of GO and time of incubation, which is not possible to compare between the studies. In addition, these studies also could not conclude if the antibacterial activity is more efficient in Gram-positive or -negative bacteria. Based on the studies, it may be concluded that the treatment conditions depend on various factors, including amount, type, size and virulence of the bacteria. It can be seen that the antibacterial activity of GO is well-established, as many studies have reported its time and concentration-dependent effects (Table 1). However, it is still unknown and inconclusive whether GO is more effective towards Gram-positive or Gram-negative bacteria. It is imperative that researchers fully understand the antibacterial effects of GO in established biological models for further development of GO in clinical settings.
Table 1. Antibacterial activity of GO reported as time and concentration-dependent.
| Findings | Concentration | Time | Reference |
|---|---|---|---|
| Results showed concentration-dependent decrease in the survival rate of K. pneumoniae, E. coli and P. aeruginosa. Antibacterial effect was most effective on K. pneumoniae. This was evident with bioluminescence indicating live bacteria. | 0–500 µg/mL | 2 h | [11] |
| The MIC for E. coli, K. pneumoniae, P. mirabilis and S. aureus is 0.065 µg/mL. The MIC for P. aeruginosa and S. marcescens is 0.032 µg/µL. The MBC of P. aeruginosa and S. marcescens is 0.065 µg/mL. The MBC for E. coli, K. pneumoniae, P. mirabilis and S. aureus is 0.12 µg/mL. | 0.004–1 µg/mL | 24 h | [12] |
| Results showed concentration-dependent increase in the zone of inhibition for E. coli and S. aureus. Zone of inhibition was bigger on S. aureus. | 250–1000 µg/mL | 24 h | [13] |
| Results showed thesignificant growth inhibition of S. aureus at 2 and 24 h and for P. aeruginosa at 2 h. | 50 mg/L | 2 and 24 h | [14] |
| MIC for E. coli and E. faecalis was 1 µg/mL and 4 µg/mL, respectively. | - | 24 h | [15] |
| Results showed that the percentage loss in viability increases as the concentration and time increases. | 12–50 µg/mL | 30–180 min | [16] |
| Results showed concentration- and time-dependent decreased viability of bacteria. The significant reduction of viability of S. aureus was 12 h, while, for E. coli, it was 168 h. | 0–40 mg/mL | - | [17] |
| After 15 min, there was 99% loss in viability of Mycobacterium smegmatis, E. coli and S. aureus. | 1 mg/mL | 15 min in the dark | [18] |
| Results showed a decrease in the recovery of E. coli. | 1 mg/mL | 2 and 4 h | [19] |
| The survival rate of E. coli is 4% at 100 µg/mL. Results also showed that the average growth delay of exponential cells varies around 4 to 6 h. E. coli and P. aeruginosa also showed alow survival rate. On the other hand, a bacteriostatic effect was observed on S. aureus, as indicated by the high survival rate. | 0–100 µg/mL | 30 min | [20] |
| Results showed time-dependent decrease in the viability of E. coli. | 0.1 g/L | 0–5 h | [21] |
| The MIC and EC50 on E. coli are 100 µg/mL and 38 µg/mL, respectively. The MIC and EC50 on S. iniae are 125 µg/mL and 29 µg/mL, respectively. | 25–150 µg/mL | 3 h | [22] |
| Results showed a time-dependent loss in viability of P. aeruginosa, and the percentage loss at 4 h is 87%. There was a concentration-dependent loss in viability that reached a complete loss at 175 µg/mL. Bacterial growth was shown to increase and decrease after 3 h at 75 µg/mL. There was a 92% growth inhibition at after 15 h. | 0–200 µg/mL | 2 h | [23] |
| Results showed the concentration- and time-dependent decreases in viability of E. coli. | 0–80 µg/mL Time study: 40 µg/mL |
2 h | [24] |
Furthermore, there has also been a handful of studies demonstrated that the effect of GO is effective both in Gram-positive and Gram-negative, according to their results. It was found that a study claimed that the thin peptidoglycan layer of the Gram-negative E. coli is easily penetrated by GO [15]. Interestingly, SEM in another showed the penetration of the cell membrane of S. aureus and claimed the antibacterial activity was more potent in Gram-positive bacteria, which has a thick peptidoglycan layer. This research also mentioned that E. coli has porins, which block the entrance of GO [13]. Another study also mentioned that the resistance of the Gram-negative E. coli is attributed to the presence of an outer membrane layer [25]. On the other hand, another study mentioned that the thickness of peptidoglycan activity does not affect the antibacterial activity of GO [26]. Various studies have shown the antibacterial activity of GO on different types of bacteria. Hence, these results are inconclusive for making a general rule on which type of bacteria is more potent in GO [27]. This has also been mentioned in a previous review discussion [28].
3. Mechanisms of Action
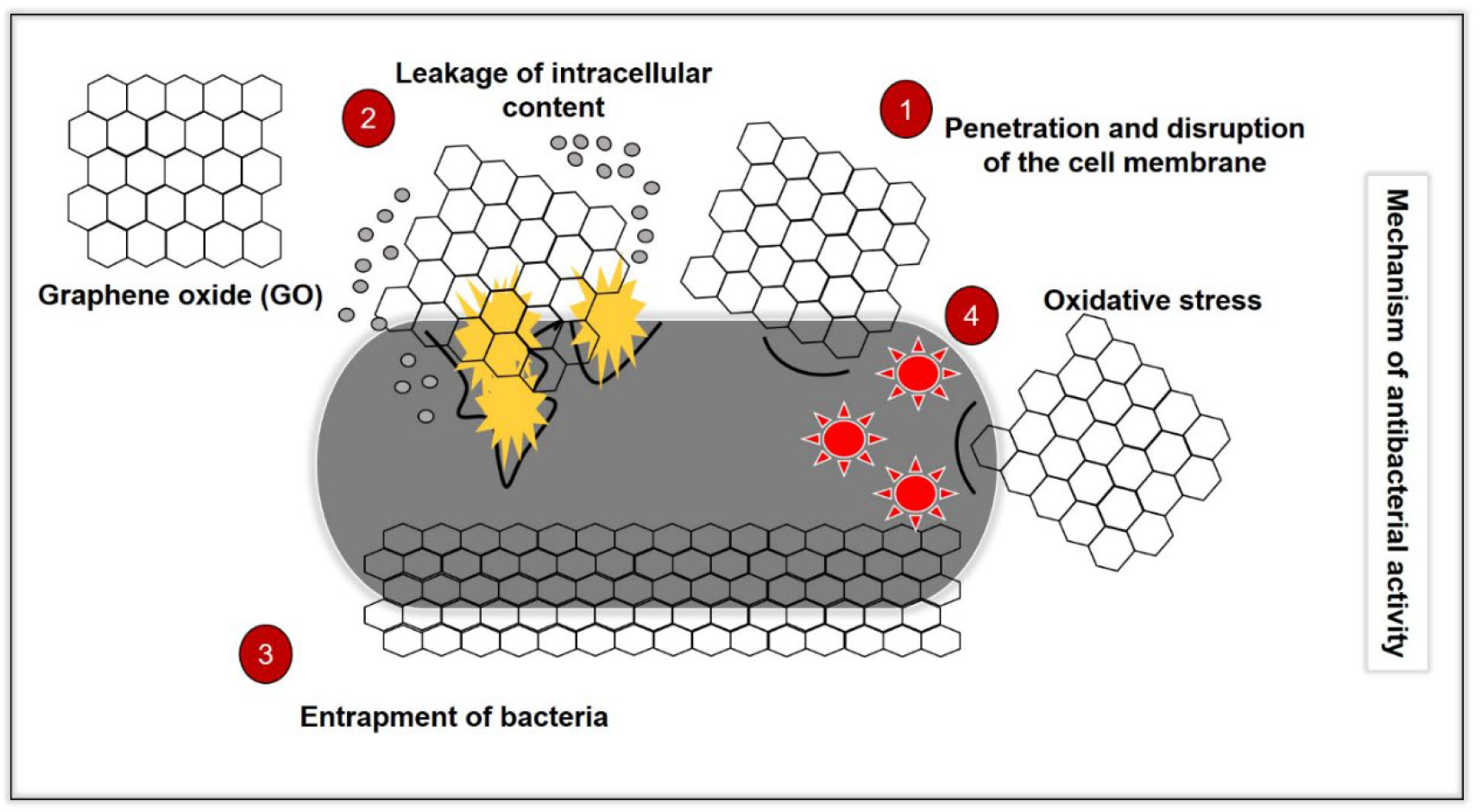
It well-known that GO demonstrates a different antibacterial mechanism than conventional antibiotics (Figure 2). The mechanisms exhibited by common MDR bacteria against antibiotics have been discussed in another review [7]. Hence, in this section, researchers will discuss the antibacterial mechanisms of GO and the limitations between the studies. This knowledge can forge the pathway for future studies on the development of GO as an antimicrobial agent.

Figure 2. Illustration of the antibacterial mechanisms of GO, which consist of (1) penetration and disruption of the bacterial cell membrane, (2) leakage of the intracellular content following penetration and disruption of the cell membrane, (3) oxidative stress by the generation of reactive oxygen species (ROS0 and depletion of the antioxidant in the bacteria) and (4) bacteria entrapping (wrapping effect) by GO.
3.1. Disruption of Bacteria Cell Membrane
A study has demonstrated the antibacterial activity of GO nanowalls against S. aureus and E. coli, which was attributed to the direct contact of the very sharp edges (nano-knives) on the nanowalls and the bacteria. In this research, SEM showed that the nanowalls that were almost perpendicular to the substrate that it was deposited on provided extremely sharp edges, allowing efficient interaction with the bacteria. The leakage of intracellular components also confirmed the cell membrane damage by the sharp edges of the GO nanowalls, which was more effective on S. aureus than E. coli. It was mentioned to be due to the lack of an outer membrane on S. aureus [25]. Interestingly, another study also showed that controlled vertically aligned GO nanosheets penetrated and disrupted the membrane of E. coli. In this research, GO nanoflakes suspension was used [29]. Thus, it is suggested that it could be due to the availability of more sharp edges in the suspension than that of being coated as GO is more freely mobile. This was supported by two other studies, which demonstrated that GO vertically oriented to the cell membrane is more ready for penetration due to it being able to overcome the energy barrier [30][31]. Moreover, studies with simulations have also reported that the GO nanosheets would enter a fluctuation or swinging mode so that they will find a diving force to overcome the energy barrier before piercing through the cell membrane [32][33]. In addition, another study also mentioned that vertical GO from approximately 3.5–4.7 nm of the bacteria membrane surface would always spontaneously penetrate through the cell membrane. Furthermore, after penetrating into the membrane, simulation experiments described the mechanism of GO disrupting the membrane by phospholipid extraction. This was said to be due to the hydrophobic interactions of the phospholipids and the hydrophobic “sp2” regions on the GO. It was also claimed that a larger GO (500 nm) demonstrated stronger antibacterial effects due to stronger cutting effects. Moreover, a larger GO also gives a larger hydrophobic region, which enhances the phospholipid extraction activity [33]. Another study also mentioned the importance of “sp2” regions in interactions with the phospholipid membrane [24]. Interestingly, SEM images from one study showed that a smaller GO (1295 nm) exhibits enhanced cutting effects on S. mutans. This is suggested to be attributed to a smaller area with more sharp edges exposed [16]. Nevertheless, 500 nm [33] is smaller than 1295 nm [16]. Another study also reported that smaller GO nanosheets enable the blade effect of sharp edges as its predominant antibacterial mechanism [34]. As mentioned above, these claims are due to researchers synthesising GO in different range of sizes. A study demonstrated that the presence of electrolytes in the GO suspension would prevent the activity of sharp edges due to reacting with the ions, forming aggregates [35]. It was said that the aggregation of GO increases the surfaces energy, thereby preventing the cutting action of the sharp edges. This research also demonstrated that the optimum concentration for the action of sharp edges is below 6 µg/mL when GO is suspended in any solution. However, as the concentration increases, antibacterial activity is only observed in GO dispersed in water. This was further confirmed by the observation of cellular debris and change in the morphology of bacteria on the AFM [34]. In addition to this, quantum mechanics calculations also indicated that further oxidation on the previous oxidised sites could significantly decrease the energy barrier [33]. In addition, another simulation study also reported that the degree of oxidation of GO affects its position within the phospholipid bilayer [31]. One other study mentioned than larger GO nanosheets with a higher degree of oxidation could cause larger and more irregular membrane perturbation [32]. In addition, another study also suggested that electron transfer from the membrane of E. coli to the oxygen functional groups of GO coated on metals leads to a loss of membrane integrity [36]. Moreover, another study also reported the availability of peaks or sharp edges promotes a charge transfer, thereby leading to destruction of the cell membrane. This was made evident by the increase in uptake of PI, a membrane impermeable dye, by the bacteria [18]. Therefore, it is important to consider these factors in the design and synthesis of GO.
3.2. Bacteria Entrapping (Wrapping) Effect
A recent study has reported that larger-sized GO have a stronger entrapping ability [16]. In this research, SEM revealed GO with larger sizes ranging from 2015 to 4544 nm exhibited an entrapping effect on S. mutans. Furthermore, an SEM observation in a previous study has shown the entrapping effect of GO (0.31 ± 0.2 µm) on E. coli [37]. In addition to this, it was also reported in another study that larger-sized GO (0.65 µm2) possessed a stronger entrapping effect on E. coli. However, this effect was only bacteriostatic [38]. These findings were also observed in previous studies. After sonication to release the bacteria from the nanoparticles, the bacteria were able to proliferate once again [39][40][41]. Interestingly, another study showed the entrapping ability of GO nanoflakes (450–870 nm) on Mycobacterium smegmatis to be bacteriostatic [42]. One other study also mentioned that larger GO (>0.4 µm2) exhibited stronger antibacterial effects on E. coli, as they have a better bacterial entrapping ability, which is observed by AFM. This research described the inhibition of nutrient uptake due to bacteria being trapped within the GO, leading to cell death [24]. A handful of studies have claimed that larger GO exhibited a stronger entrapping effect. However, the effects of the GO sizes on this mechanism are not conclusive. This could be due to several reasons, as suggested below:
-
The method of size measurement used is different (area and diameter).
-
The difference in ranges of the GO sizes. For example, one study may synthesise nanosize GO, while, in another study, microsized GO is used.
-
The different types of bacteria with different sizes and concentrations used.
One study has described the role of GO nanosheets with peaks and terrains in the bacteria-entrapping activity. It was mentioned that the entrapping effect was enhanced when the size of the bacteria matches the deep terrains of GO, which led to the degradation of the membrane integrity and intracellular leakage, as observed on the SEM [18]. Another study demonstrated the wrapping effect of GO on S. aureus but not P. aeruginosa on AFM [14]. It was speculated to be attributed to the interaction with the thick peptidoglycan of S. aureus [43]. Once again, another study also showed the stronger entrapping ability of GO on S. aureus than E. coli. It was suggested that the smaller and spherical S. aureus was easier to entrap [17]. Moreover, another study proposed the bacteria-entrapping effect as the antibacterial mechanism against various multidrug-resistant bacteria. It was suggested to be due to the similar or slightly larger size of GO, which allowed bacteria entrapment [16]. One other study also mentioned that GO nanosheets larger than the bacteria have enhanced entrapping activity. AFM observation showed no change in the morphology, which confirmed the bacteria entrapment [34]. Thus, it can be seen that the size and shape of bacteria play a role in the bacteria-entrapping mechanism.
3.3. Oxidative Stress
Oxidative stress caused by GO can be via the production of ROS of the depletion of antioxidants present in the bacterial cell [44]. One recent study has reported that the size of GO does not affect the oxidative stress activity ability on S. mutans [16]. Interestingly, a previous study demonstrated that smaller GO nanosheets coated on a nitrocellulose filter surface are better at inducing oxidative stress on E. coli [38]. This was also observed in another study [45]. This was speculated to be due to the higher defect densities on the carbon structure, which allowed more oxygen adsorption onto the GO nanosheets. [46]. This was indicated by the higher D band-to-G band ratio of Raman spectroscopy. Interesting, this ratio has a strong correlation with glutathione oxidation (R2 = 0.96). It was also mentioned that the oxidation capacity of GO nanosheets does not associate with the amount of oxygen-containing functional groups [38]. One other study also mentioned that the adsorption of oxygen on the defect sites leads to the production of ROS, eventually oxidising glutathione [47]. In addition to this, another study also reported the presence of defects leading to the formation of hydroxyl radicals. Defects were observed by the increase in the D band in the Raman analysis. It was said that the hydroxyl radicals will attack the carbonyl groups in the peptide linkages on the bacterial membrane [22]. It was mentioned that a tighter bacteria entrapping effect and presence of defects improves the interaction between the GO and bacteria, leading to a better charge transfer, increasing oxidative stress [18].
Furthermore, another study also suggested the oxidative stress-inducing activity as an antibacterial mechanism for immobilised flat GO on the PET substrate. The immobilised GO renders it unable to penetrate or entrap the E. coli. Thus, this research suggested that the oxidative stress was caused by electron transfer via the direct contact of the bacterial with the GO surface [48]. Moreover, another study demonstrated that the availability of the GO basal plane is essential for its antibacterial activity [49]. Nonetheless, a simulation study reported that further oxidation in the oxygen-containing functional groups could improve the oxidation reaction pathways [31]. Moreover, another study reported that GO coated on a metal surface is able to induce oxidative stress via ROS production against E. coli. This was said to be attributed to the electron transfer from the bacteria membrane to the oxygen functional groups, generating ROS [36]. On the other hand, it was reported in a study that physical interactions do not play a major role in the antibacterial activity of surface-coated GO. AFM has shown repulsive forces between GO and E. coli. In addition, the bridging of lipopolysaccharides on the cell surface also creates a protective barrier. In this research, GO demonstrated its antibacterial activity by the oxidation of glutathione [41]. Therefore, it can be seen that, generally, GO coated on surfaces induces oxidative stress as its main antibacterial mechanism. In contrast, several studies have also shown that ROS produced in bacterial cells exposed to GO is higher than other graphene derivatives. This was suggested to be due to the stability of GO in suspensions [28][50][51]. It was also reported that GO could easily react with water to form hydroxyl radicals, leading to a higher production of ROS [22]. In addition to this, it was also observed that oxidative stress is more effectively induced in Gram-negative bacteria. A few studies have claimed that it was due to the difference in structures of the cell membranes. For example, a study mentioned that Gram-positive bacteria are more susceptible to GO due to the lack of an outer membrane [22]. Several other studies claimed that GO is more effective on Gram-negative bacteria due to the thinner peptidoglycan layer [15][17][20]. Interestingly, another study reported that the peptidoglycan layer does not affect the antibacterial activity of GO [24]. Nevertheless, the antibacterial activity of GO does not depend on the structure of the cell membrane alone. There are other factors that contribute to the antibacterial effect, such as enzymatic activity [52].
4. Photoactivation of GO
The antibacterial activity of GO has also been reported to be induced by photoactivation. For example, one study demonstrated that GO reduced to rGO via light induction produces ROS via the singlet oxygen–superoxide anion radical pathway, which was shown to have antibacterial activity against Enterobacter sp. [53]. Another study mentioned that E. coli was able to kill itself as it reduces GO into rGO in anaerobic conditions [54]. In addition, reduced GO/ZnO nanowires were also reported to have a photo-induced antibacterial effect against E. coli. In this research, XPD data showed that GO underwent a photocatalytic reduction due to the charge transfer between GO and ZnO, although the material coated onto ZnO was just GO. Interestingly, the photoinactivation of E. coli by reduced GO/ZnO nanowires, which was 99.5%, was signficantly higher than ZnO alone, which was 58% [55]. These studies showed that the reduction of GO can be considered an intermediate pathway in its antibacterial mechanism. It was also noticed that these studies gave GO treatment at the initial stage. This could be due to the lack of dispersibility of rGO in water due to the reduction of hydrophilic functional groups.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169096
References
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as Cancer Theranostic Tool: Progress and Future Challenges. Theranostics 2015, 5, 710–723.
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; Di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential Novel Therapeutic Strategies in Cystic Fibrosis: Antimicrobial and Anti-Biofilm Activity of Natural and Designed -Helical Peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 145.
- Alsheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480.
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 23, 464–472.
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012.
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908.
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front. Microbiol. 2021, 12, 677.
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, Facts, Opportunities, and Challenges in Nanomedicine. Chem. Rev. 2013, 113, 3407–3424.
- Masoudipour, E.; Kashanian, S.; Maleki, N. A Targeted Drug Delivery System Based on Dopamine Functionalized Nano Graphene Oxide. Chem. Phys. Lett. 2017, 668, 56–63.
- Wu, X.; Tan, S.; Xing, Y.; Pu, Q.; Wu, M.; Zhao, J.X. Graphene Oxide as an Efficient Antimicrobial Nanomaterial for Eradicating Multi-Drug Resistant Bacteria in Vitro and in Vivo. Colloids Surf. B Biointerfaces 2017, 157, 1–9.
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial Activity of Graphene Oxide Nanosheet against Multidrug Resistant Superbugs Isolated from Infected Patients. R. Soc. Open Sci. 2020, 7, 200640.
- Ghanim, R.R.; Mohammad, M.R.; Hussien, A.M.A. Antibacterial Activity and Morphological Characterization of Synthesis Graphene Oxide Nanosheets by Simplified Hummer’s Method. Biosci. Biotechnol. Res. Asia 2018, 15, 627–633.
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18.
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of Antibacterial Mechanism of Graphene Oxide Using Raman Spectroscopy. Sci. Rep. 2016, 6, 28443.
- Yu, C.H.; Chen, G.Y.; Xia, M.Y.; Xie, Y.; Chi, Y.Q.; He, Z.Y.; Zhang, C.L.; Zhang, T.; Chen, Q.M.; Peng, Q. Understanding the Sheet Size-Antibacterial Activity Relationship of Graphene Oxide and the Nano-Bio Interaction-Based Physical Mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009.
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of Graphene Oxide on the Antibacterial Activity of Antibiotics against Bacteria. Environ. Sci. Nano 2017, 4, 1016–1024.
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled Surface-Mediated Antibacterial Activity of Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351.
- Barbolina, I.; Woods, C.R.; Lozano, N.; Kostarelos, K.; Novoselov, K.S.; Roberts, I.S. Purity of Graphene Oxide Determines Its Antibacterial Activity. 2D Mater. 2016, 3, 025025.
- Karahan, H.E.; Wei, L.; Goh, K.; Liu, Z.; Birer, Ö.; Dehghani, F.; Xu, C.; Wei, J.; Chen, Y. Bacterial Physiology Is a Key Modulator of the Antibacterial Activity of Graphene Oxide. Nanoscale 2016, 8, 17181–17189.
- Jayanthi, S.; Eswar, N.K.; Satyapaul, A.S.; Chatterjee, K.; Madras, G.; Sood, A.K. Macroporous Three-Dimensional Graphene Oxide Foams for Dye Adsorption and Antibacterial Applications. RSC Adv. 2015, 6, 1231–1242.
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117.
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914.
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372.
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736.
- Mokkapati, V.R.S.S.; Pandit, S.; Kim, J.; Martensson, A.; Lovmar, M.; Westerlund, F.; Mijakovic, I. Bacterial Response to Graphene Oxide and Reduced Graphene Oxide Integrated in Agar Plates. R. Soc. Open Sci. 2018, 5, 181083.
- Seifi, T.; Kamali, A.R. Anti-Pathogenic Activity of Graphene Nanomaterials: A Review. Colloids Surf. B Biointerfaces 2021, 199, 111509.
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077.
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.-H.; Osuji, C.O.; Elimelech, M. Enhanced Antibacterial Activity through the Controlled Alignment of Graphene Oxide Nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801.
- Li, Y.; Yuan, H.; Von Dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene Microsheets Enter Cells through Spontaneous Membrane Penetration at Edge Asperities and Corner Sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300.
- Wang, J.; Wei, Y.; Shi, X.; Gao, H. Cellular Entry of Graphene Nanosheets: The Role of Thickness, Oxidation and Surface Adsorption. RSC Adv. 2013, 3, 15776–15782.
- Mao, J.; Guo, R.; Yan, L.T. Simulation and Analysis of Cellular Internalization Pathways and Membrane Perturbation for Graphene Nanosheets. Biomaterials 2014, 35, 6069–6077.
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive Extraction of Phospholipids from Escherichia Coli Membranes by Graphene Nanosheets. Nat. Nanotechnol. 2013, 8, 594–601.
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria Meet Graphene: Modulation of Graphene Oxide Nanosheet Interaction with Human Pathogens for Effective Antimicrobial Therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627.
- Wu, L.; Liu, L.; Gao, B.; Muñoz-Carpena, R.; Zhang, M.; Chen, H.; Zhou, Z.; Wang, H. Aggregation Kinetics of Graphene Oxides in Aqueous Solutions: Experiments, Mechanisms, and Modeling. Langmuir 2013, 29, 15174–15181.
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149.
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980.
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236.
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288.
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity of Cellular Uptake of Cellulose Nanocrystals. Nano Life 2012, 2, 1241006.
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing Inhibitory Effects of Nanocrystalline Cellulose: Inhibition versus Surface Charge. Nanoscale 2012, 4, 1373–1379.
- De Maio, F.; Palmieri, V.; Salustri, A.; Perini, G.; Sanguinetti, M.; De Spirito, M.; Delogu, G.; Papi, M. Graphene Oxide Prevents Mycobacteria Entry into Macrophages through Extracellular Entrapment. Nanoscale Adv. 2019, 1, 1421–1431.
- Castrillón, S.R.-V.; Perreault, F.; De Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117.
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2011, 25, 15–34.
- Liu, Y.; Wen, J.; Gao, Y.; Li, T.; Wang, H.; Yan, H.; Niu, B.; Guo, R. Antibacterial Graphene Oxide Coatings on Polymer Substrate. Appl. Surf. Sci. 2018, 436, 624–630.
- Liu, X.; Sen, S.; Liu, J.; Kulaots, I.; Geohegan, D.; Kane, A.; Puretzky, A.A.; Rouleau, C.M.; More, K.L.; Palmore, G.T.R.; et al. Antioxidant Deactivation on Graphenic Nanocarbon Surfaces. Small 2011, 7, 2775–2785.
- Koch, G.; Yepes, A.; Förstner, K.U.; Wermser, C.; Stengel, S.T.; Modamio, J.; Ohlsen, K.; Foster, K.R.; Lopez, D. Evolution of Resistance to a Last-Resort Antibiotic in Staphylococcus aureus via Bacterial Competition. Cell 2014, 158, 1060–1071.
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; De Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the Antibacterial Mechanism of Graphene Oxide (GO) Langmuir–Blodgett Films. Chem. Commun. 2015, 51, 2886–2889.
- Hui, L.; Piao, J.G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the Basal Planes of Graphene Oxide Determines Whether It Is Antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190.
- Sodhi, N. New Mechanism of Resistance in a Last-Resort Antibiotic. Aust. Vet. J. 2016, 94, N8–N9.
- Pumera, M. Graphene-Based Nanomaterials and Their Electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157.
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028.
- Dutta, T.; Sarkar, R.; Pakhira, B.; Ghosh, S.; Sarkar, R.; Barui, A.; Sarkar, S. ROS Generation by Reduced Graphene Oxide (RGO) Induced by Visible Light Showing Antibacterial Activity: Comparison with Graphene Oxide (GO). RSC Adv. 2015, 5, 80192–80195.
- Akhavan, O.; Ghaderi, E. Escherichia Coli Bacteria Reduce Graphene Oxide to Bactericidal Graphene in a Self-Limiting Manner. Carbon 2012, 50, 1853–1860.
- Nourmohammadi, A.; Rahighi, R.; Akhavan, O.; Moshfegh, A. Graphene Oxide Sheets Involved in Vertically Aligned Zinc Oxide Nanowires for Visible Light Photoinactivation of Bacteria. J. Alloys Compd. 2014, 612, 380–385.
This entry is offline, you can click here to edit this entry!
