Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
The effects materialize in economic losses, alterations of the quality and organoleptic properties of the commercial products, and, last but not least, they constitute risk factors for the consumer’s health.
- metallic nanoparticles
- metallic oxides
- biosensors
1. The Types of Bio-Nanosensors Used in the Food Industry
Food contamination with pathogenic microorganisms or associated toxins can cause acute and chronic diseases and can lead to epidemics and death. Pathogenic bacteria exist in a variety of shapes and types and could be highly heat resistant (e.g., Clostridium botulinum, C. perfringens, Bacillus subtilis, B. cereus), could be capable of producing heat-resistant toxins (e.g., Staphylococcus aureus, Clostridium botulinum), or could grow under temperatures of less than 10 °C or in refrigerated conditions [108]. Due to the diversity of foodborne pathogenic agents and their rapid spread, recent studies reported advances in the development of nanosensor-based technologies, such as nanobiosensors, DNA biosensors, smartphone-based biosensors [109].
Food nanotechnology can be applied in order to increase food production, as well as for the development of food with higher nutritional value and quality. However, despite the numerous recent research works in this area, there still exist numerous challenges and opportunities to improve the current sensor technology. Sensing food pathogen development will be useful for quality control; consumers will know that products are compliant, and the frequency of food-borne diseases will be reduced. A typical biosensor (Figure 1) contains bio-recognition elements, such as enzymes, antibodies, proteins, cells, the analyte, such as toxins, bacteria, enzymes, antibodies, proteins, cells, and a transducer, able to convert biological response into measurable electronic signals. Digital electronic signals are directly related to the target biomolecules’ concentration [110].
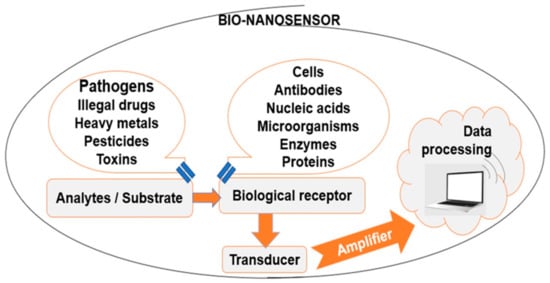
Figure 1. Typical bio-nanosensor components.
As a function of the transducer type, biosensors can be classified as in Figure 2.
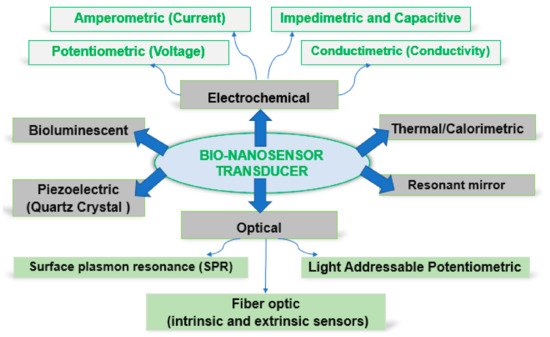
Figure 2. Schematic representation of biosensor technologies.
Therefore, nanosensors are used to transmit the information about nanoparticles to the macroscopic world. The development of nanomaterials in the form of nanoparticles, nanofibers, nanowires, or nanotubes has found applications as bio-detectors and bio-analyzers, being capable of very fast detection, even of a single cell of foodborne pathogens. The use of very low nanoparticle doses successfully detected foodborne infections [111]. Rapid detection can be carried out through optical sensors that work on light scattering technology, which can differentiate bacterial colonies up to the genus, species, and strain level, as in the case of Listeria, Staphylococcus, Salmonella, Vibrio, and Escherichia coli [112,113,114]. For specific recognition of pathogens, metal, and magnetic nanoparticles [114], polymeric [115] or inorganic semiconductors have been conjugated with biologically sensitive elements (called receptors), such as antibodies, enzymes [116], antibiotics, nucleic acids [117], lipids, tissue, or microorganisms [118,119].
2. Use of Metallic and Metal Oxides Nanoparticles for Sensing Food Pathogens
2.1. Nanoparticles Application in Food Pathogen Sensing Technologies
In the past decades, numerous research works [120,121,122] discussed the potential of nanoparticles (NPs) and their incorporation into biosensor systems in order to achieve the use of NPs in biosensor applications for the detection of food pathogens. Nanotechnology-based detection methods use metallic NPs, such as gold and silver [123], with tunable size and shape.
The metallic nanoparticles can be used as sensing platforms for the target pathogen (such as Escherichia coli, Staphylococcus aureus, Salmonella typhymurium, Enterococcus faecalis, Aspergillus niger, Fusarium oxysporum) from different food matrices, generating a combined response pattern selective for every pathogen [124,125]. The response will be quantified by different sensing platforms, such as colorimetric chemosensors. Nanostructures have the potential to be used as simple and good sensing elements in a colorimetric sensor system due to their functions of receptors and indicators simultaneously, leading to color change. The mechanism of metallic nanoparticles (leading to color change)—electrochemical–surface plasmon resonance sensor (EC-SPR)—phytosynthesized nanomaterials, can be used as coating for the sensing electrode modification for identification of the bacterial pathogens in clinical and food-related samples [16,126].
The simplest method of detection based on nanotechnology is the colorimetric detection. For example, the interaction/binding between gold nanoparticles and analytes represents an interaction that induces the aggregation of gold nanoparticles and the subsequent visual change of color from red to blue. Biodetection based on the localized surface plasmon resonance (LSPR) band modification is a method based on changing the spectral characteristics induced by a variation in the local environment of gold nanoparticles, following biospecific interactions [127]. This method is less sensitive than the aggregation tests, and since any molecule can induce a change in the LSPR band of gold nanoparticles, the method is only suitable for the detection of known analytes and only when gold nanoparticles are functionalized with molecular recognition elements, such as antibodies [128]. For the detection and identification of unknown samples, Raman spectroscopy is the most appropriate method because it is a very specific technique able to identify molecular species based on their unique Raman vibration fingerprint. The Raman signal can be drastically amplified when the analytes are adsorbed on metal surfaces, an effect known as surface-amplified Raman scattering. The surface-enhanced Raman scattering (SERS) substrate is therefore essential for efficient biodetection applications. In solution, the aggregated or anisotropic nanospheric gold nanoparticles cause the formation of hot spots between the interconnected nanoparticles that substantially amplify the Raman signal. As important as the form of gold nanoparticles is the functionalization and biocompatibility of the sensors that must allow the adsorption of the analytes for an efficient, stable, and reproducible SERS signal [129,130].
E. coli O157:H7 presence in food determined serious issues for human health and financial losses for food producers. Meng Xu et al. [131] developed a rapid biosensor for E. coli O157:H7 detection in pure culture and in food. They obtained bifunctional polymeric nanocomposites (PMNCs), which contained antibodies (ABs-rabbit anti-E. coli O + K polyclonal antibodies) and glucose oxidase (GOx). Firstly, magnetic beads (MBs) were linked to GOx by the streptavidin–biotin reaction, followed by obtention of a thin film of polydopamine (PDA) on MB-GOx by dopamine (DA) self-polymerization. The AuNPs were biochemically synthesized on the MBs-GOx@PDA PMNCs through the in situ reduction in chloroauric acid by the H2O2. As stated by the authors, the use of biocompatible PDA allowed the GOx to maintain its enzymatic activity to catalyze glucose to produce H2O2. ABs and GOx adsorption measurement led to ABs/GOxext/AuNPs/MBs-GOx@PDA PMNCs, which was used to separate the pathogenic agent from the food and to amplify the signal. Mixing the target bacteria capture method with labeling steps, these bifunctional PMNCs with AuNPs proved short detection time. Additionally, the transfer of the biological recognition to the signal took place because the numerous linking sites on the bacteria had abundant and active enzymes attached. Regarding the E. coli O157:H7 capture efficiency, the PMNCs with AuNPs captured about 89% bacteria cells between 102 and 105 CFU/mL. Considering about 10% bacteria losses, the PMNCs proved a good efficacy. The properties of this prepared biosensor demonstrated its efficacy in sensing food pathogens. The AuNPs were obtained using the method previously presented by Fu et al. [132]. Fu et al. [132] developed performant polymeric bionanocomposites (PBNCs) containing PDA, Pt nanoparticles (PtNPs), GOx, AuNPs, and an antibody (antihuman immunoglobulin) by in situ synthesis of NPs. PBNCs exhibited high enzymatic activity, useful in signal improvement, and high antibody loading for efficient immuno-recognition.
Zhongyu Fu et al. [133] outlined a rapid AuNPs-based colorimetric assay for detection of Listeria monocytogenes and Salmonella enterica, using the PCR method and thiol-labeled primers (PCR being applied to amplify the hly gene of L. monocytogenes and the hut gene of S. enterica). AuNPs (13 nm in diameter) were prepared by the citrate reduction in HAuCl4 and exhibited a characteristic surface plasmon band centered at 520 nm. Mixing the products with thiol label (as PCR results) and AuNPs, the sulphur–gold linkage resulted. These are more tolerant of salt than primers linked to AuNPs, so colorimetric testing using naked eye or spectrophotometric measurements could be used for pathogenic bacteria detection in food.
Xiaolin Huang et al. [134] reported a homogeneous AuNPs-based immunoassay using dynamic light scattering (DLS) for detection of Listeria monocytogenes (L. monocytogenes) from lettuces. Having a large surface (0.5 μm × 2.0 μm), L. monocytogenes possess numerous antigen epitopes, which act as binding carriers for the AuNPs, leading to AuNPs-coated bacteria complexes. In their research study, various parameters were changed until optimized development conditions were established and also to separate and concentrate L. monocytogenes from lettuce samples. Regarding the sensitivity and reproducibility of the AuNPs-based DLS immunoassay, NPs with three different diameters were obtained and used (small, medium, and large). Small-sized AuNPs were synthesized using the Li et al. method [135], and medium and large sizes were obtained using another method [136] based on hydroquinone (HQ). When large AuNPs (100 nm) were used, the limit of detection (LOD) for L. monocytogenes in lettuce probes reached 2.2 × 101 CFU/g. Their values were much lower than the maximum limit imposed in Canada (100 CFU/g). The procedure was applied to 17 common pathogenic bacteria, but the results clearly demonstrated that the best results were recorded for L. monocytogenes detection.
Miranda et al. [137] developed a sensitive colorimetric enzyme-AuNPs sensing for detection of bacteria. The AuNPs functionalized with quaternary ammonium ligands are electrostatically bound to β-galactosidase (β-Gal), leading to the inhibition of enzymatic activity without denaturation. Upon exposure to the bacteria, the functionalized NPs bind to their anionic surface, releasing the enzyme, which turns the pale-yellow substrate to red. This method demonstrated, in the case of E. coli (XL1), a visual sensitivity of 1 × 104 bacteria/mL, but for other bacteria, the sensitivity may vary depending on the bacteria species.
In recent years, NPs-based LSPR biosensors have been studied for their efficiency in the recognition of bacteria [138]. In the case of some LSPR biosensors with a low detection limit, the incorporation of AuNPs and an aptamer was studied. In 2017, Seo Yeong Oh et al. [139] proposed a portable plasmonic biosensor for Salmonella typhimurium detection in artificially contaminated pork meat. Large-area AuNPs were immobilized on the glass substrate, and the aptamer was bound to the AuNPs by a dipping process. Firstly, AuNPs with controlled size were synthesized as Neus G. Bastus et al. described [140]. Secondly, a glass substrate treated for impurities was removed and then covered with 0.5% 3-aminopropyl)-triethoxysilane (APTES) as a linker with the amine group, which was prepared. Finally, the amino-functionalized substrate was immersed into an AuNPs solution for 8–9 h, and the change from colorless to burgundy demonstrated the adhesion of AuNPs onto the glass substrate. The obtained LSPR sensing devices exhibited a detection limit of 104 CFU/mL in pure culture and also in artificially contaminated pork meat probes, the S. typhimurium being identified using an aptamer as a linker between the AuNPs-conjugated system and bacteria.
A highly sensitive and rapid method for colorimetric detection of Bacillus cereus (B. cereus) in milk was developed by coupling asymmetric PCR (producing long genomic DNA fragments from the cereulide synthetase gene, cesB) with propidium monoazide (PMA) and unmodified AuNPs [141]. Under optimum conditions, the LOD for viable emetic B. cereus in phosphate-buffered saline (PBS) and milk was 9.2 × 101 CFU/mL and 3.4 × 102 CFU/mL, respectively. According to the results, this method was appropriate to respect the maximum limit established by the Commission Regulation (EC) No 2073/2005 (500 CFU/mL) and is considered a good detection test for food-borne pathogens.
The simultaneous detections of Shigella boydii (S. boydii) and Escherichia coli O157:H7 (E. coli O157:H7) in three types of food products using the colloidal gold immunochromatographic strip (ICS) was reported [142]. The pathogen agent detection from food products was evaluated by strip tests and the ELISA method. The LOD by the strip tests reached 106 CFU/mL for both food-borne pathogens, without any cross-reaction with other related bacteria. The LOD was enhanced to 4 CFU/mL when all three food products containing S. boydii and E. coli O157:H7 were pre-incubated.
In 2013, Yun Ju Sung et al. [143] prepared a sensing mechanism for Staphylococcus aureus detection in milk. The resulting sensing method based on a composite antibody/AuNPs/carboxylated magnetic nanoparticle demonstrated high capture efficiencies for S. aureus in PBS of 96% and 78% in milk. Using the properties of both the magnetic nanoparticle and the AuNPs, such as magnetic separation and signal generation, the LOD of this colorimetric sensor in PBS for S. aureus reached 1.5 × 103 CFU and 1.5 × 105 CFU in milk.
A variety of sensing devices are also based on silver NPs (AgNPs) [144,145]; this type of NPs presents some disadvantages, such as NPs degradation leading to Ag+ ions caused by functionalization and oxidation phenomenon appearing on the surface of AgNPs. A very efficient immunosensor for S. aureus detection was fabricated by Abdolkarim Abbaspouret et al. [146]. For a system assay in the form of a sandwich, the following components were used: a biotinylated anti-S. aureus primary aptamer fixed on magnetic beads (MB) that were coated with streptavidin for sample capture, an anti-S. aureus secondary aptamer conjugated with AgNPs [147,148] for increasing signal, and implicit for target bacteria detection, and an electrochemical stripping voltammetry read-out for sensitive detection. The obtained immunosensor exhibited a dynamic range from 10 to 1 × 106 CFU/mL and a LOD of 1.0 CFU/mL.
The Escherichia coli detection with surface-enhanced Raman scattering (SERS) was achieved by Naja et al. [149] using polyclonal antibodies on protein-A-modified AgNPs. In their experimental data, Escherichia coli ATCC 13529 and Rhodococcus rhodochrous ATCC 17895 (American Type Culture Collection, Manassas, VA, USA) were used, and the results showed that, although the two bacteria were present, only E. coli was absorbed by the AgNPs. The proposed Raman scattering technique and also attaching an appropriate polyclonal antibody to the target bacteria on the nanoparticles surface assured specific and more selective bacteria detection results as compared to the traditional Raman spectroscopy. SERS with silver nano substrates were used for detection and identification of three food-borne pathogens (E. coli O157: H7, S. aureus, and Salmonella) investigated by Wei et al. [150]. For the silver colloidal NPs synthesis, the microwave heating method was applied. The rapid SERS procedure [151] combined with silver colloidal nanoparticles, which play an important role in the signal enhancement for SERS, proved high reproducibility for all three studied food-borne pathogens. In 2013, Cowcher et al. [152] used SERS for rapid and sensitive detection of Bacillus in food, mixing silver colloidal nanoparticles with the bacteria. The use of SERS techniques [153] for food-borne pathogen detection in food is constantly evolving. This led to the use of different types of metal nanoparticles (MNPs) and metal oxides (MOs) [154], such as copper oxide (CuO), titanium dioxide (TiO2), Zinc oxide (ZnO), or silver oxide (Ag2O), due to their promising properties in food industries. CuNPs [155] can be used to detect pathogenic agents responsible for food spoilage. Being less expensive, Cu and copper oxide (CuO) are more attractive compared to noble metals. TiO2 nanocrystals, prepared as D. C. Pan et al. described [156], were used as optical nanomaterials for spectroscopic detection of Salmonella in milk [157]. A polyclonal antibody-conjugated magnetic nanoparticles complex captured Salmonella bacteria under an external magnetic field. The resulting magnetic nanoparticle/Salmonella was re-dispersed in PBS and exposed to antibody-immobilized TiO2NPs, exhibiting an increase in light absorption. Combining the immuno-magnetic separation with optical nanomaterials for Salmonella detection, a TiO2NPs-based immunoassay was obtained as a good alternative to the traditional methods based on PCR. The limit of detection for the targeted bacterium in milk was higher than 100 CFU/mL. When ZnO-NPs with special electronic configuration are used, the actions of the released Zn2+ ions are very important. The contact of ZnO-NPs with the pathogen surface determined the formation of electrostatic forces affecting the cell membrane [16]. Some examples of metallic nanoparticles applied in sensing food pathogens are presented in Table 1.
Table 1. Food-borne pathogen, predominant symptoms, and the metallic nanoparticles used for sensing food pathogens.
| Target of Assay | Predominant Symptoms |
Type of NP-Based Biosensor |
Biosensing Element | Detection Limit | Ref. |
|---|---|---|---|---|---|
| E. coli | Diarrhea, abdominal pain, nausea, vomiting | AuNPs (flower-shaped, F-AuNPs and sphere-shaped, S-AuNPs) | In situ reaction between NPs and specific primer (target gene wzy) | 50 pg/μL E. coli 0157:H7 |
[158] |
| E. coli | Diarrhea, abdominal pain, nausea, vomiting | AuNPs | Aptamers against ethanolamine and E. coli O111:B4 lipopolysaccharides | 1 µg/mL E. coli | [159] |
| Listeria monocytogenes | Headache, fever, chills | AuNPs (flower-shaped, F-AuNPs and sphere-shaped, S-AuNPs) | In situ reaction between NPs and specific primer (target gene hly) | 10 pg/μL; | [160] |
| Listeria monocytogenes | Headache, fever, chills | Phytosynthesized flower-shaped AuNPs | NPs hybridized with primers (target genes hlyAF and hlyAR) | 100.4 ng | [161] |
| Salmonella enterica | Fever, abdominal cramps, diarrhea, vomiting | AuNPs | Biotinylated rabbit anti-Salmonella polyclonal antibody | 104 CFU/mL in PBS and 105 CFU/mL in milk |
[158] |
| Salmonella typhimurium | Fever, abdominal cramps, diarrhea, vomiting | AuNPs (flower-shaped, F-AuNPs and sphere-shaped, S-AuNPs) | In situ reaction between NPs and specific primer (target gene hut) | 10 pg/μL | [160] |
| Salmonella typhimurium | Fever, abdominal cramps, diarrhea, vomiting | AgNPs | Cationic AgNPs functionalized with anti-Salmonella antibody | 102 cells/mL | [162] |
| Bacillus cereus | The emetic and diarrheal syndrome, abdominal cramps | AuNPs | AuNP-based colorimetric assay combined with asPCR amplification and propidium monoazide treatment | 9.2 × 101 CFU/mL in 0.01 M phosphate-buffered saline and 3.4 × 102 CFU/mL in milk | [141] |
| Campylobacter jejuni | Fever, arthralgia, chills, headache, | Gold-Palladium nanoparticles (Au@Pd) | NPs covered with specific DNA aptamer () | 100 CFU/mL | [163] |
| Staphylococcus aureus | Nausea, abdominal pain, vomiting, diarrhea, | Gold and iron oxide (Fe3O4/Au) nanoparticles | Etching-enhanced peroxidase-like catalytic activity of gold nanoparticles | 10 CFU/mL | [164] |
| Shigella spp. | Fever, abdominal cramps, diarrhea, vomiting | AuNPs | Aptamer coated NPs | 80 CFU/mL Shigella flexneri | [165] |
| Aptamer surface functionalized composite material containing an Eu-complex and AuNPs | 10 CFU/mL Shigella sonnei | [166] | |||
| Yersinia enterocolitica O:8 strains | Abdominal pain, diarrhea, fever | AuNPs | Monoclonal antibody labeled AuNP | 5 CFU/mL in milk and pork samples | [167] |
| Trichinella spiralis | Gastroenteritis, fever, muscular pain | AuNPs | anti-rabbit polyclonal antibody conjugated AuNPs | - | [168] |
| Clostridium botulinum | Respiratory paralysis, double or blurred vision, loss of light reflex | AuNPs | Immobilization of cleaved SNAptide with cysteine ends onto AuNPs via the thiol group | 0.25 ng/mL | [169,170] |
| Staphylococcus aureus | Nausea, abdominal pain, vomiting, diarrhea, | AuNPs | Aptamer (anti S. aureus immunoglobulin Y) modified NPs | 10 FCU/mL | [147] |
| Pseudomonas aeruginosa | The emetic and diarrheal syndrome, abdominal cramps | AuNPs | Aptamer (P. aeruginosa-specific aptamer F23) modified NPs | 60 CFU/mL | [171] |
| Aspergillus niger | Asthma or other chronic lung diseases | AuNPs | AuNPs conjugated with thiol-containing fungal spore-binding peptide ligands | ∼>50 spores | [172] |
| Candida | Oral thrush, pseudomembranous, erythematous (atrophic) and hyperplastic | Fe3O4NPs, AgNPs | Fe3O4NPs/polyethylenimine composites captured Candida, while positively charged AgNPs were used as SERS substrate | - | [173,174] |
2.2. Functionalization of Metallic Nanoparticles for Sensing Food Pathogens
The development of successful bio-sensors for food pathogens sensing systems, as can be seen from the examples provided in the previous paragraphs, can be achieved by several different approaches.
- a.
-
Direct interaction of the nanoparticles and the analytes, inducing a visible color change and a corresponding spectral LSPR change. This is the simplest approach, in which the colloidal NPs solution is mixed with a solution containing the analyte. The spectral change can be easily followed by UV-Vis spectrometry. The approach was presented in several studies, being used for the detection of Listeria monocytogenes and Salmonella enterica (as demonstrated by Zhongyu Fu et al. [133]) or for the detection of emetic B. cereus [141] and Bacillus spores [152] in milk samples.
- b.
-
Physical deposition of NPs on different substrates represents another viable alternative. It can be achieved either by direct synthesis on the surface of different materials (as demonstrated by Xu et al. [131] and Fu et al. [132]) or by the deposition of the NPs on substrates (for example, on glass substrates coated with (3-aminopropyl)-triethoxysilane), as presented by Oh et al. [139].
- c.
-
The coating of metallic nanoparticles with specific antibodies was also presented in several studies. For example, Huang et al. [134] obtained anti-Listeria monocytogenes mAbs-coated AuNPs by adding the antibodies solution to the AuNPs solution, under magnetic stirring, further blocked with polyethylene glycol and bovine serum albumin; using the same method, anti-clenbuterol monoclonal antibody/AuNPs (further deposited on paper strips) [135] or double monoclonal antibodies (against Shigella boydii and Escherichia coli O157:H7) conjugated gold nanoparticles (also deposited on test strips) were obtained.
- d.
-
The functionalization of metallic nanoparticles was presented in several works. For example, Miranda et al. [137] obtained cationic AuNPs by obtaining pentanethiol-coated AuNPs (using a two-phase synthesis method), which were further quaternary-ammonium functionalized by the Murray place-exchange method and further deposited on test strips. Magnetic nanoparticles were functionalized with glutaraldehyde (in order to form amine groups and amine-reactive crosslinkers on the NPs surfaces), on which monoclonal Salmonella antibodies were further immobilized [157].
All the approaches presented have their particular advantages and disadvantages. However, in order to pass from the laboratory scale to practical day-by-day application, the biosensor needs to be simple to use and to reveal the presence of the food contaminants. One such approach is represented by the incorporation of biopolymers to develop smart packaging materials [175]. This would allow the development of easy-to-use sensing technologies, which would provide a visual indicator for the final consumer. Although this approach is very promising, there remains the issue of nanoparticle migration into the products, which raises health issues and remains to be addressed via toxicity studies.
This entry is adapted from the peer-reviewed paper 10.3390/ma15155374
This entry is offline, you can click here to edit this entry!
