Electrolyte-insulator-semiconductor (EIS) field-effect sensors belong to a new generation of electronic chips for biochemical sensing, enabling a direct electronic readout. The review gives an overview on recent advances and current trends in the research and development of chemical sensors and biosensors based on the capacitive field-effect EIS structure—the simplest field-effect device, which represents a biochemically sensitive capacitor. Fundamental concepts, physicochemical phenomena underlying the transduction mechanism and application of capacitive EIS sensors for the detection of pH, ion concentrations, and enzymatic reactions, as well as the label-free detection of charged molecules (nucleic acids, proteins, and polyelectrolytes) and nanoparticles, are presented and discussed.
- chemical sensor
- biosensor
- field effect
- capacitive EIS sensor
- pH sensor
- enzyme biosensor
- label-free detection
- charged molecules
- DNA biosensor
- protein detection
1. Functioning Principle of Capacitive EIS Sensors
Figure 1a schematically shows a typical layer structure of a capacitive EIS sensor and a simplified electrical equivalent circuit. The EIS sensor consists of a semiconductor substrate (in this case, p-type silicon) separated from the solution by a thin (10–100 nm) gate insulator layer (or stack of layers) and a rear-side contact layer (e.g., Al). The gate insulator is assumed to be ideal—that is, no current passes through the insulator. For the operation of EIS sensors, a gate voltage (VG) is applied between the reference electrode (RE, e.g., conventional Ag/AgCl liquid-junction electrode) and the rear-side contact to regulate the capacitance and set the working point; a small alternating voltage (~10–50 mV) is superimposed to measure the capacitance of the structure. For a proper measurement, the reference electrode should provide a stable potential independent of the pH value of the solution or concentration of the dissolved species.
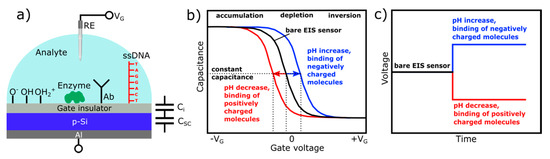
Figure 1. Layer structure of a capacitive electrolyte-insulator-semiconductor (EIS) sensor with different receptor functionalities (pH-/ion-sensing, enzyme, antibody, and DNA) and simplified electrical equivalent circuit (a); typical shape of high-frequency capacitance-voltage (C–V) curves (b); and ConCap response (c) of the bare and modified p-type EIS sensor. RE: reference electrode, VG: gate voltage, Ab: antibody, DNA: deoxyribonucleic acid, Ci: gate-insulator capacitance, CSC: space-charge capacitance, ssDNA: single-stranded DNA.
2.Measurement Modes of Capacitive EIS Sensors
The electrical equivalent circuit of the EIS sensor is complex and involves components related to the semiconductor, gate insulator, electrolyte/insulator interface, bulk electrolyte, and reference electrode [1][2]. However, for the usual range of a gate insulator thickness and appropriate experimental conditions used (electrolyte solution with ionic strength of >0.1 mM and measurement frequencies of <1 kHz), the equivalent circuit of an EIS sensor can be simplified as a series connection of the gate-insulator capacitance, Ci, and the variable semiconductor space-charge capacitance, Csc (VG,φ), which is, among others, a function of the gate voltage, VG, and the electrolyte–insulator interfacial potential, φ [2]. Hence, the expression for the total capacitance, C, of the bare EIS sensor is given in Equation (1):
EIS sensors are basically characterized by means of the capacitance-voltage (C–V) and/or constant-capacitance (ConCap) mode [3][4]. The typical shape of a high-frequency C–V curve for a p-type EIS sensor with characteristic regions of accumulation, depletion, and inversion is exemplarily shown in Figure 1b (black curve); note, an n-type EIS sensor exhibits an identical C-V curve; however, the voltage polarity is reversed. If a negative potential (VG < 0) is applied to the gate, the positively charged holes (majority carriers) will be attracted and accumulated at the semiconductor/insulator interface. In accumulation regime, Ci << Csc(VG,φ), i.e., the overall capacitance of the EIS structure is determined by the geometrical capacitance of the gate insulator, C = Ci, and, thus, corresponds to its maximum capacitance.
When applying a small positive potential (VG > 0) to the gate, the holes will be pushed away from the interface semiconductor/insulator. As a result, a space-charge region is formed at the semiconductor/insulator interface, which is depleted of mobile carriers (so-called depletion region). The width of the depletion layer is determined by different parameters, such as the applied voltage, doping concentration within the semiconductor, dielectric constant, and insulator thickness. Increasing the amplitude of the applied gate voltage results in an increase of the width of the depletion layer and, consequently, to a decrease of the total capacitance. If the magnitude of the positive gate potential is sufficiently high, the Fermi level bends below the intrinsic level: the concentration of electrons near the semiconductor/insulator interface exceeds the hole concentration, i.e., a thin layer of n-type silicon (so-called inversion layer) is formed, although the substrate is a p-type. By strong inversion, the width of the depletion layer reaches its maximum, and the high-frequency total capacitance of the EIS structure approaches its minimum value.
Equation (1) describes the total capacitance of the EIS sensor without defining the origin of the potential generation at the interface electrolyte/insulator. When fixing the applied gate voltage, VG, the only variable component is the interfacial potential, φ, which is analogous to the effect of applying an additional voltage to the gate. Since FEDs are potential-/charge-sensitive devices, any kind of chemical and/or electrical change at or nearby the interface electrolyte/gate can be detected by the EIS sensor. Those changes can be induced by biochemical reactions, when the capacitive field-effect sensor is functionalized with a particular chemical and/or biological recognition element, such as a pH-sensitive layer, ionophore, enzyme, antibody, nucleic acid, etc. For (bio-)chemical sensor applications and for investigating charge effects in such capacitive EIS structures, the shift of the C–V curves along the voltage axis (ΔVG) in the depletion region (Figure 1b) is more important. The direction of these potential shifts depends on the charge sign of the adsorbed chemical and/or biological species. For example, in case of a p-type EIS structure, an increase of the analyte’s pH value or binding of the negatively charged species to the gate surface will decrease the width of the depletion layer, yielding an increase of the depletion capacitance in the Si. By this, the total capacitance of the sensor will increase, and the C–V curve will shift to the direction of more positive (or less negative) gate voltages (Figure 1b, blue curve). Conversely, a pH decrease or the electrostatic adsorption or binding of positively charged species to the gate surface will lead to an increase of the width of the depletion layer; the space-charge capacitance will decrease. As a consequence, the total capacitance of the EIS sensor will also decrease, resulting in a shift of the C–V curve towards more negative (or less positive) gate voltages (Figure 1b, red curve).
The amplitude, as well as the direction of potential shifts, can directly be determined from dynamic ConCap-mode measurements (see Figure 1c). In addition, the ConCap mode enables real-time monitoring of the sensor signal and investigation of the response time, drift, and hysteresis of the EIS sensor. In the ConCap mode, the total capacitance of the EIS sensor at the working point is kept constant by using a feedback circuit, which applies an instantly sign-inverted voltage to the EIS sensor. Usually, the working point for the ConCap mode is set within the linear range of the depletion region of the C–V curve.
In the following sections, an origin of different mechanisms of interfacial potential generation (e.g., pH and ion-concentration changes, enzymatic reactions, adsorption, and binding of charged molecules and nanoparticles) is described, which enables EIS devices to be sensitive to numerous chemical and biological species, as well as to discuss the physicochemical phenomena underlying the transduction mechanism of EIS-based chemical sensors and biosensors.
This entry is adapted from the peer-reviewed paper 10.3390/s20195639
References
- Fabry, P.; Laurent-Yvonnou, L. The C-V method for characterizing ISFET or EOS device with ion-sensitive membranes. J. Electroanal. Chem. 1990, 286, 23–40.
- Poghossian, A.; Ingebrandt, S.; Abouzar, M.H.; Schöning, M.J. Label-free detection of charged macromolecules by using a field-effect-based sensor platform: Experiments and possible mechanisms of signal generation. Appl. Phys. A 2007, 87, 517–524.
- Klein, M. Characterisation of ion-sensitive layer systems with a C (V) measurement method operating at constant capacitance. Sens. Actuators B 1990, 1, 354–356.
- Schöning, M.J.; Brinkmann, D.; Rolka, D.; Demuth, C.; Poghossian, A. CIP (cleaning-in-place) suitable “non-glass” pH sensor based on a Ta2O5-gate EIS structure. Sens. Actuators B 2005, 111–112, 423–429.