Myocarditis comprises many clinical presentations ranging from asymptomatic to sudden cardiac death. The history, physical examination, cardiac biomarkers, inflammatory markers, and electrocardiogram are usually helpful in the initial assessment of suspected acute myocarditis. Echocardiography is the primary tool to detect ventricular wall motion abnormalities, pericardial effusion, valvular regurgitation, and impaired function. The advancement of cardiac magnetic resonance (CMR) imaging has been helpful in clinical practice for diagnosing myocarditis. The present review highlights the unmet clinical needs for uniform CMR criteria for diagnosing acute and chronic myocarditis in children and differentiating dilated chronic myocarditis phenotype from idiopathic dilated cardiomyopathy.
1. Introduction
Myocarditis is an inflammatory disease of the myocardium and is a rare diagnosis in children accounting for only 0.05% of pediatric hospital admissions [
1]. Acute myocarditis has a heterogeneous clinical course ranging from the asymptomatic presentation and gradual onset of heart failure to fulminant myocarditis presenting as cardiogenic shock and sudden death [
2,
3]. The complexity of these divergent presentations is further compounded by the continuously evolving diagnostic criteria, making the diagnosis exceptionally challenging [
2]. Children with acute myocarditis often present with resting tachycardia, chest pain, respiratory distress, abdominal pain, and vomiting [
3,
4,
5,
6]. Chest pain is a typical symptom of acute myocarditis in adolescents [
7]. Electrocardiography (ECG) abnormalities are present in approximately 90% of children with myocarditis, including ST-T wave, changes, low voltage QRS complexes, and atrioventricular conduction delays [
3,
5,
6]. Elevations in cardiac biomarkers such as troponin and brain-type natriuretic peptides are common but not specific for acute myocarditis in children [
3,
7,
8].
Echocardiography is the primary tool to determine the presence of left ventricular (LV) wall motion abnormalities, pericardial effusion, valvular abnormalities, and function assessment [
9]. Endomyocardial biopsy (EMB) is the “gold standard” to confirm myocarditis, but it is an invasive procedure with suboptimal sensitivity. The advancement of cardiac magnetic resonance (CMR) imaging has been helpful in clinical practice for diagnosing myocarditis. Reflecting this evolution, a recent Scientific Statement by the American Heart Association (AHA) suggested CMR as the current gold standard for diagnosing myocarditis [
10]. In addition to tissue characterization, CMR is useful for evaluating the ventricular function, myocardial wall thickness, chamber dilation, and pericardial effusion.
Clinically acute myocarditis implies a short time elapsed from the onset of symptoms (generally <1 month and up to 8 weeks) [
11,
12]. Chronic myocarditis (course of disease ≥ 3 months) is a type of inflammatory cardiomyopathy with either a dilated or non-dilated phenotype that is often characterized by regional or global wall motion abnormalities and impaired LV function [
10]. In general, the prognosis of acute myocarditis is good, but up to 30% of cases may progress to develop a dilated cardiomyopathy phenotype [
13]. The relative roles of viruses, host genomics, and environmental factors in disease progression and recovery are still unknown [
14]. Consequently, treatment strategies are not well established. Confirming the diagnosis of acute myocarditis in children by CMR with tissue characterization using inverse recovery acquisition findings of myocardial inflammation is an essential milestone in the right direction and a paradigm shift towards a decreased need for EMB [
10]. However, standard CMR parametric mapping parameters for diagnosing myocarditis are not established in pediatric patients for consistency and reliability in the interpretation.
2. CMR Findings of Myocardial Inflammation and Pathological Correlations
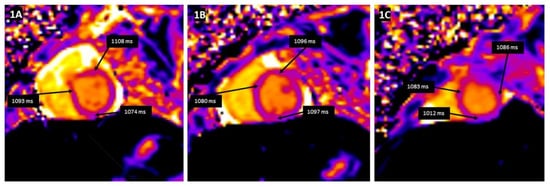
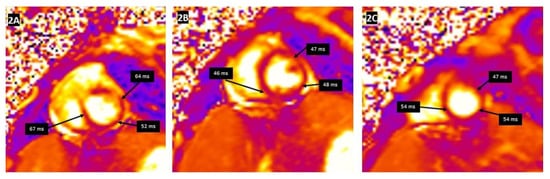
Tissue characterization by CMR parametric mapping allows quantifying myocardial changes based on T1 and T2 images for fibrosis, water content, and extracellular volume fractions (ECV), a surrogate of interstitial fibrosis (
Figure 1A–C and
Figure 2A–C). It enables differentiation between normal myocardial muscle, edema, and fibrosis, thereby serving as a virtual biopsy [
14,
15]. CMR allows evaluation of all myocardial segments, contributing to its superior sensitivity to EMB to detect myocarditis because myocardial inflammation often has a heterogeneous, “patchy” distribution. Late gadolinium enhancement (LGE) helps detect scarring and necrosis.
Figure 1. T1 mapping demonstrates a global increase in myocardial T1 relaxation times at the base (A), mid-ventricular level (B), and the apex (C). The average left ventricular myocardial T1 relaxation time is prolonged (1067 ms), and myocardial extracellular volume is elevated (32%).
Figure 2. T2 mapping demonstrating the regional increase in myocardial T2 relaxation times at the basal anteroseptal and anterolateral segments (A), with normal T2 times at the mid-ventricular level (B) and the apex (C). Clinical vignette: A 20-month-old female with myocarditis associated with rhino/enterovirus, rapid left ventricular ejection fraction recovery from 24% to 48%, elevated troponin, and diffuse low voltage QRS complexes on electrocardiogram. There was no evidence of myocardial delayed enhancement after gadolinium contrast emphasizing the additional utility of T1 and T2 mapping in the evaluation of myocarditis.
The diagnostic CMR criteria are derived from the common consensus among experts (Lake Louise Consensus (LLC) criteria) for myocarditis in adults [
16]. The hallmark features of myocardial inflammation on CMR are (1) T2-weighted imaging, which assesses for edema; (2) T1-weighted early gadolinium enhancement imaging (EGE), which assesses for hyperemia; and (3) LGE, which assesses for myocyte necrosis and scar. The CMR LLC criteria seem to have moderate accuracy in diagnosing acute myocarditis [
17,
18]. LLC criteria are qualitative analyses and rely upon the increased signal intensity of the myocardium relative to the normal myocardium. These criteria, therefore, have limited applicability for diffuse myocardial involvement without focal findings. Subsequently, a revised version of the LLC was released in 2018 with quantitative tissue mapping techniques. According to the updated LLC criteria, myocarditis can be diagnosed with high specificity based on: (i) the presence of one of the T2-based criteria (i.e., regional or global increase in T2 relaxation time or increased signal intensity on T2 weighted images) and (ii) the presence of one T1-based criterion out of three: prolonged T1 relaxation time, elevated ECV or LGE [
19]. Both criteria have a reported median area under the curve for detecting myocarditis ranging from 0.75 to 0.90. Either isolated T1- or T2-based criteria in the appropriate clinical setting can suggest myocardial inflammation in adults [
19,
20]. It is important to note that the LLC criteria have not been validated in children.
The parametric mapping techniques have only recently begun to enter clinical use in children, and their predictive utility in acute myocarditis remains an active area of investigation. There is no comprehensive data for CMR based on the revised LLC criteria in pediatric myocarditis except for small observational studies. A study on 43 consecutive clinically suspected pediatric acute myocarditis patients demonstrated that the revised LLC criteria enhance the diagnostic performance compared to conventional LLC criteria [
21]. In another retrospective study in 58 children with acute clinical myocarditis, LGE was identified as a predictor of poor outcomes [
22]. Conventional LGE imaging can underestimate the amount of myocardial injury in myocarditis. Native T1 and ECV maps may reveal hidden myocardial injury in the normal-appearing myocardium of patients with myocarditis.
In one study, CMR was reported to have a low yield in diagnosing the severity of acute myocarditis and does not predict outcomes in children [
23]. However, this study was likely underpowered to detect the outcome. Due to variability between individual scanners and local site references, there are significant differences in CMR tissue characterization techniques among pediatric centers and no standardized cutoff values for T1 or T2 relaxation times. Most centers use LGE for diagnosing scar or myocardial necrosis [
24]. The presence of LGE is likely a robust prognostic marker in children and adults with myocarditis [
25,
26].
Subepicardial inferolateral LGE is the most common pattern in adult patients with acute myocarditis [
27]. In contrast, a higher prevalence of a mid-wall or mixed LGE pattern is reported in pediatric patients [
27]. The mid-wall or mixed pattern of LGE was associated with more severe disease progression and a higher complication rate than the subepicardial inferolateral pattern [
28,
29]. The LGE distribution pattern may help differentiate myocarditis from ischemic myocardial injury as seen in the anomalous left coronary artery from the pulmonary artery (ALCAPA), congenital coronary ostial stenosis or atresia, coronary fistula, or Kawasaki disease (KD) [
30,
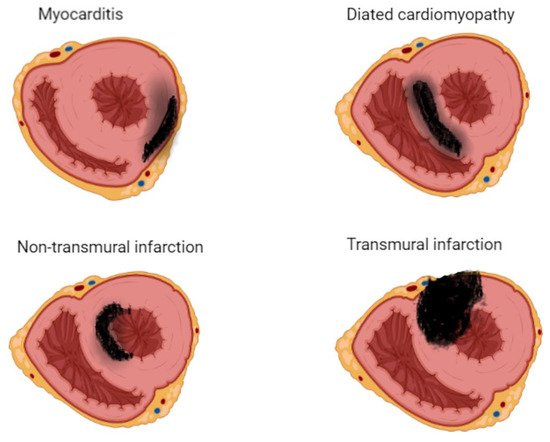
31]. LGE involves the subendocardium following a coronary territory in cases of ALCAPA, and KD confers to an infarction-like pattern. In more severe cases, LGE may extend to transmural, resulting in a fully transmural pattern (
Figure 3).
Figure 3. Late gadolinium enhancement (LGE) imaging allows the noninvasive visualization of areas affected by myocardial scar, conferring clinicians the unique ability to differentiate ischemic from nonischemic lesions based on typical LGE patterns.
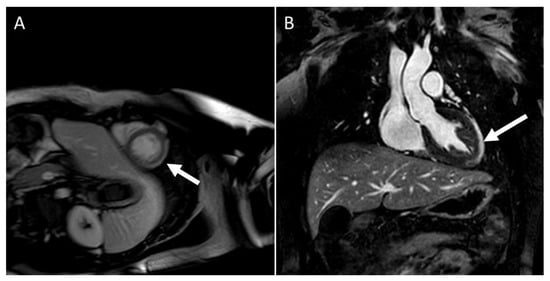
LGE patterns for viral myocarditis are often confined to the intramural septal wall or subepicardial lateral wall (
Figure 4A,B), but the presence of LGE alone does not inform the chronicity of the disease process. Furthermore, Mahrholdt et al. [
32] described different LGE patterns and correlated them with viral etiologies of acute myocarditis in children and young adults. Parvovirus B19 myocarditis had LGE in the inferolateral segments. In contrast, human herpes simplex type 6 (HHV6) myocarditis had LGE in the intraventricular septum, and the difference in distribution was due to different viral cardiac tropisms. A summary of CMR findings in children with either clinically suspected or EMB proved acute and chronic myocarditis in children are described in
Table 1.
Figure 4. CMR in an adolescent with acute myocarditis: (A) Shows subepicardial early gadolinium enhancement (EGE) (white arrow); (B) Shows subepicardial LGE (white arrow).
Table 1. CMR findings in clinically suspected acute and chronic myocarditis in children.
Patients with DCM often have a linear mid-myocardial LGE pattern, but a subendocardial pattern strongly suggests previous myocardial ischemia or necrosis due to microthrombus but spontaneous coronary recanalization or distal embolization from minimally stenotic coronary lesions [
33]. In contrast, chronic myocarditis rarely presents with a subendocardial LGE pattern and rarely follows coronary artery distribution. Instead, the hallmark LGE pattern of chronic myocarditis consists of patchy or longitudinal striae of mid-wall and subepicardial enhancement. In chronic myocarditis, parametric tissue mapping to evaluate myocardial T1 relaxation time and ECV helps detect diffuse fibrosis without focal LGE [
34].
It is important to differentiate between inflammatory cardiomyopathy (subacute/chronic myocarditis with abnormal ventricular function) from idiopathic dilated cardiomyopathy as there is a significant difference in prognosis and outcomes between the two. In chronic myocarditis, the sensitivity of CMR drops significantly as edema becomes progressively less prominent than in acute myocarditis and even disappears entirely in up to 84% of cases. Furthermore, long-term studies are necessary to determine whether CMR can assist in predicting long-term outcomes.
This entry is adapted from the peer-reviewed paper 10.3390/children9071061