Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Among the biomolecules of emerging scientific interest are antimicrobial peptides (AMPs), potent biomolecules that can potentially act as important weapons against infectious diseases. Moreover, synthetic AMPs are easily tailored (bioinformatically) to target specific features of the pathogens to hijack, inducing no or very low resistance.
- antimicrobial peptides
- antimicrobial treatment
- infection
1. Increasing Interest in New Bioactive Antimicrobial Peptides
During the COVID-19 pandemic, the search for antimicrobial bioactive compounds assumed greater proportions compared with prepandemic periods. New biomolecules can potentially act as important weapons against infectious diseases, while their variability offers an arsenal of possibilities still little explored. Among the biomolecules of emerging scientific interest are antimicrobial peptides (AMPs) [30,31].
AMPs can be considered a new class of therapeutic agents that tackle the challenge of pathogen invasion, having several properties that make them particularly attractive, such as their small size, fast activity, and low chance of resistance development by the pathogenic targets [32,33,34,35,36,37]. They are evolutionarily ancestral molecules that evolved in living organisms over 2.6 billion years ago [37] that played a fundamental role in the evolutionary success of multicellular organisms. Such molecules remain effective weapons in organism defense against a wide variety of pathogens, including bacteria, fungi, viruses, and protozoa [33,38,39,40,41]. AMPs are small multifunctional peptides, part of the innate immunity produced by both complex organisms (eukaryotes, such as humans, animals, plants, and fungi) and prokaryotic microorganisms (bacteria) [37]. In humans and mammals, AMPs act as effectors of innate immunity on skin and mucosal surfaces [42]. Most AMPs are cationic, although also anionic peptides have been described. Cationic AMPs mainly target the microbial membrane, while anionic ones generally present intracellular targets (e.g., ribosomes) [43].
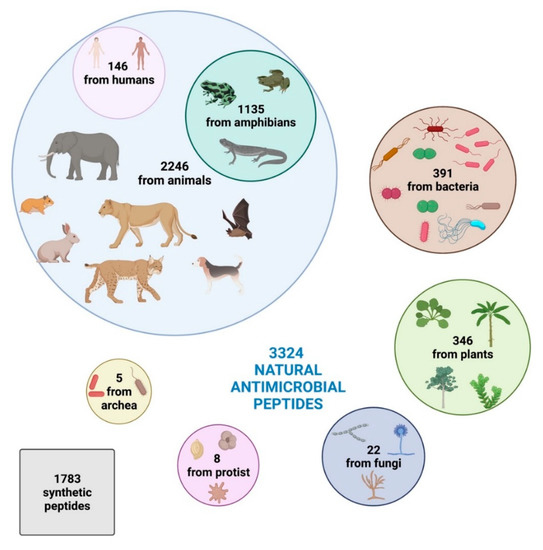
Intriguingly, based on the Antimicrobial Peptide Database [44], 3324 natural antimicrobial peptides have been identified so far and registered in the online database, divided into 2446 from animals (comprehending some synthetic peptides), 391 bacteriocins/peptide antibiotics from bacteria, 346 from plants, 22 from fungi, 8 from protists, and 5 from archaea.
Humans harbor 146 AMPs, while amphibians present the highest diversity, producing 1135 active peptides [44]. Plants, due to lack of mobile immune cells and adaptive immune response and their sessile habit that prevents them from evading adverse environmental situations, have 346 AMPs against pathogens [44].
Moreover, according to the Data Repository of AntiMicrobial Peptides (DRAMP) database [45], 1783 synthetic peptides have been developed and registered (Figure 1).
Nevertheless, vegetable diversity is still little explored with respect to the broader knowledge regarding human and animal AMPs [32,33,38,39,40,41]. Among plants, the extremophile ones, being well adapted to resist extreme environmental conditions and capable of defending themselves against different pathogenic attacks, are the most interesting, and thus represent a powerful source of defense molecules, producing snakins, heveins, α-hairpinin, and lipid transfer proteins (LTPs) [38,41,46].
AMPs have diverse and complex antimicrobial activities, showing a wide range of antiviral, antibacterial, and antifungal properties and modes of action [36].
An additionally important immunomodulatory activity in humans has been also reported through which endogenous AMPs activate the host’s immune system [36,47], an effect also attributed to when exogenous, synthetic, and vegetable AMPs are delivered.
The mechanisms of antibacterial action can be divided into membrane and nonmembrane targeting.
The first one is characterized by the formation of membrane pores, leading to the loss of intracellular ions and metabolites, depolarization, osmotic shock, and cell death [48]. It is characteristic of the cationic AMPs. Indeed, the cationic charge allows the interaction between the peptides and plasma membrane components, which results in the accumulation of these molecules on the surfaces. Anionic AMPs can also target the membrane through the exploit of metal ion cofactors, forming cationic salt bridges linked to the negatively charged membrane components of microorganisms [49].
Three models of action mechanisms have been proposed for cationic AMPs and are graphically displayed in Figure 2. In the barrel-stave model, AMPs aggregate and form a hole with the hydrophilic domains in the lumen, while hydrophobic domains come in contact with the lipid bilayer. In the toroidal pore model, AMPs enter perpendicularly the membrane, dragging and bending the lipids as they form a ring hole [50].
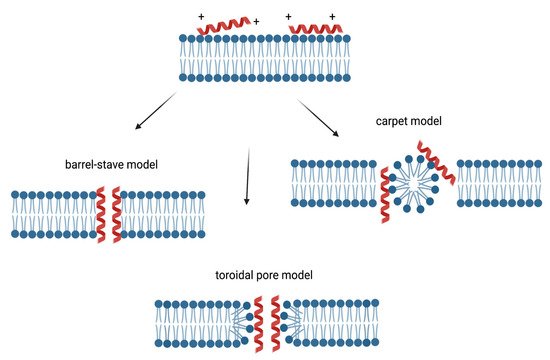
Figure 2. Membrane targeting mechanism of action of cationic AMPs. The cationic charge allows the interaction between the peptides and plasma membrane, resulting in the accumulation of these molecules on the surfaces. In the barrel-stave model, AMPs aggregate and form a hole with the hydrophilic domains in the lumen, while hydrophobic domains come in contact with the lipid bilayer [50]. In the toroidal pore model, AMPs enter perpendicularly the membrane, dragging and bending the lipids as they form a ring hole [50]. In the carpet model, AMPs can act like detergents by locating at the level of the plasma membrane, causing alterations, followed by destruction [51].
In addition, in the carpet model, AMPs can act like detergents by locating at the level of the plasma membrane, causing alterations, followed by destruction [51]. In Figure 2, the membrane targeting mechanisms of action of cationic AMPs are displayed.
In the nonmembrane mechanisms of action, AMPs enter the cells with a direct penetration or endocytosis. Once inside the cells, AMPs may have different targets. AMPs can affect the transcription, translation, and protein folding through the interference with involved effector enzymes or molecules. It has been shown that AMPs, predominantly anionic AMPs, can directly target ribosomes [52] and chaperons [53]. AMPs can also inhibit protease activity, as serine protease, elastase, and chymotrypsin [53], or target and degrade DNA or RNA, inhibiting key enzymes of nucleic acid biosynthesis but also interfering with DNA replication and nucleic acid damage response [54,55,56]. Finally, AMPs (e.g., Histatin 5) can target fungal mitochondria, leading to reactive oxygen species generation and cell death [57].
AMPs’ activity against fungi partially reflects the antibacterial mechanisms of action, through the interaction with membranes and formation of pores, as well as other specific antifungal properties, such as the targeting of the wall components and related synthase enzymes or nucleic acid, causing DNA binding or intercalation [58].
Against parasites, AMPs display similar processes by recognizing anionic phospholipids on the membrane, therefore causing osmotic imbalance and cellular shrinkage. Besides, they can also impact intracellularly producing mitochondria alterations or disruption of lysosome after endocytosis, leading to cell death [59].
Antiviral action mechanisms are different due to the high diversity of pathogenic viruses. AMPs can directly act on the virion particles targeting external viral structural proteins, inhibiting adsorption or penetration, or they can interfere inside the host cells, hindering viral proteases (essential for viral replication) or impeding viral uncoating and release [60].
Besides natural AMPs, synthetic AMPs (SAMPs) have recently emerged as a new exciting possibility of drug discovery. An AMP with affinity for a bacterial, fungal, or viral protein can not only be identified with the aid of computational strategies but also be further optimized by peptide engineering techniques to make the binding molecule more specific against a molecular target of a pathogen. Hence, based on the sequence of natural AMPs and with the help of such techniques, it is possible to develop SAMPs.
Based on AMP sequences retrieved from public databases, SAMPs can be easily developed with specific characteristics that achieve the required final effect by employing different approaches (e.g., the selection of the known functional motives, site-direct modification/mutation, or de novo construction methods) [43,61].
2. Potentiality of Synthetic Antimicrobial Peptides against Zoonoses
Despite undeniable scientific advances, pathogenic diseases continue to raise significant concerns due to their evolutionary and adaptive capacity. Consequently, the search for novel bioactive biomolecules using different mechanisms of antimicrobial action has been revitalized, gaining more attention given the current pandemic scenario.
SAMPs have been previously exploited in the challenge against zoonosis. In the following sections, some examples of the already proven efficacy of SAMPs are presented.
2.1. Viral Zoonosis
SAMPs can inhibit or interfere with different mechanisms of infection or replication of SARS-CoV-2, the betacoronavirus responsible for COVID-19, affecting primarily the respiratory system [65].
The θ-defensin analog RC101 is effective against SARS-CoV-2 pseudoparticles in vitro when the treatment was performed prior to virus inoculation. Moreover, RC101 possesses an antiviral effect against replication-competent SARS-CoV-2. These peptides partially act when administered to cells prior to pseudovirus infection but not postinfection. Further assays reveal that they mainly affect viral fusion and entry, possibly with a direct impact on the virions, but not the binding of the virus to ACE2 [66].
Several recent studies were also conducted in silico by performing molecular docking.
A good example was presented by Ling et al. [68], who designed SAMPs by molecular dynamics simulation, aiming to bind in the spike protein fusion region of SARS-CoV-2 (HR1 and HR2 regions) with the peptide derived from the spike itself. The binding energy of the predicted HR-2-derived peptide was more substantial than the natural stage of the fusion core, suggesting that the predicted antiviral peptide may competitively bind to HR1 to prevent the formation of the fusion core.
In a similar approach, Mahmud et al. [69] used computational predictive tools to select four potential candidates (out of an AMP database) with the ability to inhibit the main protease of SARS-CoV-2 (Mpro). The selected peptides (circulin A from Chassalia parviflora, piscidin 4 from Morone chrysops x/ Morone saxatilis, neutrophil defensin 1 from Pan troglodytes (chimpanzee), and corticostatin-3 from Oryctolagus cuniculus (rabbit)) bind to the active cavity of Mpro, in addition to the substrate binding sites (domain 2 and domain 3). The authors suggest that these AVPs may inhibit the Mpro of SARS-CoV-2 even though more laboratory experiments are needed to confirm such results.
An analysis of more than 50 peptides was performed computationally in order to verify the binding capacity to the receptor-binding domain (RBD) of the S protein of SARS-CoV-2. Of these, 15 peptides showed a higher affinity for the human ACE2 receptor. Two of the most promising candidates (S2P25 and S2P26) blocked the entry of SARS-CoV-2 by molecular dynamics simulation tests. Such findings may facilitate the rational design of peptides able to selectively inhibit the action of the SARS-CoV-2 S protein [70].
Zika virus (ZIKV) causes the ZIKV fever, a relatively mild illness (infected individuals present fever, joint pain, headache, cutaneous rash), but in a minority of the cases, it can result in the Guillain–Barré syndrome, a neuropathy affecting the peripheral nervous system and producing muscle weakness, which can be vertically transmitted, producing microcephaly in fetuses [71].
He et al. [72] tested several peptides derived mainly from human and bovine cathelicidins to determine their anti-ZIKV activity. Two AVPs (GF-17 and BMAP-18) stood out by showing strong in vitro activities against infected cells. These peptides effectively inhibited ZIKV, regardless of whether they were added before or after infection, suggesting that their mechanism of action is through direct virus inactivation and via the interferon pathway.
Indeed, cathelicidin derivatives have gained prominence in the search for an anti-ZIKV peptide. Xing et al. [73] designed an analog peptide from a snake venom, cathelicidin-30 (ZY13), capable of inhibiting ZIKV in vitro and in vivo. Studies regarding the mechanism of action revealed that ZY13 could directly inactivate ZIKV and reduce the production of infectious virions, in addition to strengthening the host’s antiviral immunity through the cytokine signaling protein suppressor pathway. The authors point to ZY13 as a promising anti-ZIKV drug candidate, highlighting the potential of animal venom peptides as models for developing AVPs.
The dengue virus (DENV) is a significant and growing public health problem worldwide. According to a WHO survey, approximately 400 million people are infected annually. Although most of the infected individuals are asymptomatic, approximately a quarter of them develop a wide range of clinical manifestations with unclear pathogenic mechanisms [74]. To date, there is no specific antiviral drug available to treat DENV infection. In turn, the licensed dengue vaccine (CYD-TDV or Dengvaxia®) presents some limitations for use, especially for children under 9 years of age [75].
HS-1, a synthetic peptide derived from anuran Hypsiboas semilineatus was tested in vitro against DENV serotypes 2 and 3, showing that HS-1 was active against DENV when the peptide and the viruses were cotreated and then inoculated to the cells, as well as when the binding and internalization assay was performed, while there was no impact when HS-1 was added post-infection or the cells were treated prior to viral inoculation. Interestingly, the cotreatment induced destabilization of the viral envelope, and it was also efficacious in vivo, when the treated viruses were used to infect mice [76].
Reported targets of peptide inhibitors against DENV infection generally include viral structural proteins, such as C, prM, and E, as well as viral NS2B-NS3 protease and NS5 methyltransferase [77]. For instance, Tambunan and Alamud [77] designed seven peptides against the NS2B-NS3 protease of dengue virus type 2 (DENV-2) to block active sites of viral proteins or to mimic specific regions of viral proteins as competitive inhibitors of viral entry and viral replication. In addition to the regions cited as promising targets, Songprakhon et al. [78] studied the NS1 protease as a novel peptide target to inhibit DENV. AVPs exhibited varying degrees of inhibition towards different DENV serotypes. Given the promising results, the authors suggest that these peptides could be the start of developing an inhibitor-based project for the multifunctional NS1 protein.
Another currently critical viral disease involves the outbreak of influenza A H3N2. Influenza viruses figure as an example of viruses whose evolution is highly associated with their ability to establish and spread in different host species. They have most likely originated from aquatic birds. Their evolution and ecology have been associated with various native reservoirs, from wild birds to poultry and to various mammalian host species [79].
H3N2 is currently spread in different world regions. Between July and November 2020, an influenza A (H3N2) epidemic occurred in Cambodia and other neighboring countries in the Greater Mekong subregion of Southeast Asia [80]. However, the virus mutated again in Australia this year (Darwin variant), enough to increase emergency room and hospital admissions. H3N2 was recently spread in Brazil together with an outbreak of the Omicron variant of SARS-CoV-2, resulting in many hospitalizations and deaths [81].
The influenza A virus presents a high mutation capacity, requiring a new vaccine for each new variant.
Studies by Li et al. [82] showed that the fish-skin-derived SAMP peptide P acts as a potential natural inhibitor of influenza A neuraminidase in MDCK cells in the early stage of the infectious cycle. Molecular docking simulation indicated that the SAMP could directly bind neuraminidase, being a competitive inhibitor. In the in vitro assays, peptide P was able to protect the cells from infection and to reduce virus replication. The major effect was obtained when SAMP was added prior to viral inoculation, while a minor impact was observed when it was supplemented during or post-viral absorption. These data suggest that peptide P may target the cell surface and prevent virus binding to the host cell, and the hemagglutination (HA) assay confirmed these results.
2.2. Bacterial Zoonosis
SAMPs can also be used as a valid strategy for targeting bacterial zoonoses.
Foodborne zoonotic infections impose a great threat to human consumers. Although in Europe in 2020, 120,946 notified cases of campylobacteriosis, 52,702 of salmonellosis, 4446 of STEC, 5668 of yersiniosis, and 1876 of listeriosis were reported, the real numbers may be, indeed, higher [16]
Campylobacteriosis, commonly transmitted by contaminated avian meat, is caused by Campylobacter species (mainly C. jejuni, C. coli, and C. lari), and is characterized by diarrhea, abdominal pain, nausea, and fever [83].
Talukdar et al. showed that puroindoline A (PinA) peptides derived from puroindolines (Triticum aestivum) displayed a strong antibacterial activity against Campylobacter jejuni, inhibiting its growth by disrupting its cellular membrane, while also blocking biofilm formation [84].
Salmonellosis, typically, provokes stomachache and diarrhea, but other symptoms include vomiting, nausea, fever, and muscular/articular pain. The transmission occurs through the ingestion of eggs and pig meat as well as cattle and dairy products. Despite the existence of different Salmonella serotypes, the nontyphoid ones (S. Typhimurium, S. enteritidis, S. newport, and S. Heidelberg) are those responsible for food contamination [85].
Cap-18 (from rabbit neutrophils, analog to the human LL-37) is an AMP with activity against Salmonella typhimurium in vitro. A library of 696 Cap-18 derivatives (each with a single amino acid substitution of the original peptide sequence) was screened against Salmonella typhimurium, showing that about 82% of the molecules tested presented the same activity as the original AMP, but 5 of them displayed a higher effect. The screening resulted in identifying the key amino acid residues for the antimicrobial activity of Cap-18. Moreover, the authors assayed the library against the beneficial Lactococcus lactis to determine the species specificity of the peptides [86].
Talukdar et al. [84] showed that puroindoline A (PinA) peptides derived from puroindolines (Triticum aestivum) are effective in counteracting the growth and biofilm formation of Salmonella enterica serovar Typhimurium.
Yersinia enterocolitica is the causative agent of yersiniosis. The stability of Yersinia enterocolitica in cool conditions (4 °C) and its ability to produce thermostable toxins are problematic features for humans consuming contaminated food. The classical manifestations of yersiniosis are fever, stomachache, and bloody diarrhea, mimicking appendicitis. The infection can be transmitted through pork, milk and dairy derivatives, plants, seafood, and water ingestion [85].
Sijbrandij et al. [87] tested SAMPs derived from bovine lactoferrin against Yersinia enterocolitica, finding a strong bactericidal effect due to permeabilization and depolarization of the bacterial membrane. Moreover, SAMPs inhibited also the bacterial host cell invasion (in HeLa cells) by inducing inflammatory mediators released by the HeLa cells [88]
Listeria monocytogenes causes listeriosis and is a Gram-positive bacterium able to withstand unfavorable conditions, such as 0–45 °C of temperature and a 4.4–9.4 pH range. Listeria monocytogenes are intracellular bacteria that penetrate intestinal cells, producing gastrointestinal symptoms (vomiting, stomachache, diarrhea, weariness), in addition to the cells of the spleen, liver, brain, and heart. Most cases required hospitalization with a mortality rate of 20–30% [85]. Although this pathogen is commonly found in the environment, it is, in fact, also present in domestic animals (cattle goats, horses, poultry, fishes) through which it is transmitted to humans by alimentation.
Talukdar et al. [84] found that Puroindoline A (PinA) is able to disrupt the membranes of Listeria monocytogenes, therefore blocking their expansion and the biofilm development.
Shiga-toxin-producing Escherichia coli (STEC) is a bacterium carrying the genes for the expression of Shiga toxin types 1 (Stx1) and 2 (Stx2). STEC infection causes bloody diarrhea, nausea, headache, vomiting, and abdominal cramping, with a fatality rate of 5% in children and a risk of 15% of developing hemolytic uremic syndrome and life-threatening renal failure. Transmission mainly occurs through the consumption of beef, water, and milk [89].
Lino et al. [90] showcased the antimicrobial effect of the d-amino acid hexapeptide WRWYCR against STEC. The peptide inhibited bacterial DNA repair through the strong binding to the Holliday junction intermediates blocking the resolution. Acidic stress decremented the STEC survival; nevertheless, if a pretreatment with the peptide was performed, a major reduction was achieved. The most effective protocol, where no detectable survival was measured, was formed by room temperature treatment of the bacteria with the peptide, followed by acidic treatment at 37 °C, possibly suggesting a clinical pre-ingestion approach combined with the subsequent exposure to the gastric acidic environment. Finally, the authors detected no increase in Shiga toxin production in the acidic condition.
2.3. Fungal zoonosis
Fungal infections have gained notoriety as an alarming problem in recent decades given the evolution of intrinsic resistance to current antifungals [91]. For Cryptococcus neoformans, a human fungal pathogen that mainly affects immunocompromised individuals, reports of resistance to usual therapeutics are limited [92]. Data from the Leading International Fungal Education (LIFE) website in 2017 (updated in 2018) estimate around 223,100 annual cases of cryptococcal meningitis in the immunocompromised, with a mortality rate of 10% and greater than 70% in the US and Africa, respectively, leading to approximately 181,000 deaths per year [93,94].
In this context, intensive research has been carried out using antifungal peptides (AFPs) against a diversity of fungi due to their efficacy and high selectivity [95]. Zhang et al. [96] tested an AFP, known as SP1 and derived from Saccharomyces cerevisiae, that showed potent activity against C. neoformans and C. gattii. Unlike most AFPs that form pores in the pathogen’s cell membrane, SP1 interacts with the pathogen’s membrane ergosterol and enters the vacuole, possibly through natural membrane traffic, causing calcium ion homeostasis imbalance, increased reactive oxygen, exposure to phosphatidylserine, and nuclear fragmentation. Finally, the authors suggest that SP1 has the potential to be developed as a treatment option for cryptococcosis.
Specht et al. [97], in a recent publication, demonstrated the effects of a potential peptide vaccine against cryptococcosis (C. neoformans). This peptide with 32 amino acids was able to strongly bind to the major histocompatibility complex class II (MHC II) H2-IAd allele in BALB/c mice. Therefore, the authors conclude that peptide-based vaccines containing a single peptide can protect mice against cryptococcosis. However, given the diversity of human MHC II alleles, a single-peptide-based vaccine would be challenging for human use and would likely require multiple peptide sequences.
Another disease that has gained importance in recent years due to its worldwide prevalence, distribution, and epidemiology is sporotrichosis (caused by species of fungi of the genus Sporothrix) [98,99]. This disease has different clinical manifestations (cutaneous, lymphocutaneous, and disseminated) and could also progress to a systemic infection [100].
Sporotrichosis is more prevalent in tropical and subtropical countries. Despite this, it has been reported in the United States, Europe (where cases have been reported intermittently in countries such as France, Italy, Spain, Portugal, the United Kingdom, and Turkey), Asia (China, India, and Japan), Africa (South Africa, Zimbabwe, Nigeria, and Sudan), and Australia [98].
Yan et al. [101] selected an AFP (ToAP2) from a database with the aid of bioinformatics tools and constructed three more peptides (ToAP2A, ToAP2C, and ToAP2D) using peptide design techniques. The three derived AFPs inhibited the growth of Sporothrix globosa, among which ToAP2D had the best results, displaying strong antifungal activity, good serum stability, and no acute toxicity. Scanning electron microscopy analysis revealed membrane deformation and rupture as the main mechanism of action. Overall, the authors suggest that ToAP2D is potentially therapeutic against sporotrichosis.
2.4. Zoonotic parasites
Toxoplasmosis is caused by Toxoplasma gondii, an apicomplexan parasite capable of infecting any nucleated cell in any warm-blooded animal. Cats can harbor Toxoplasma gondii, and they can be a source of animal–human transmission. About 2 billion people are infected annually; however, a small percentage of them suffer from the severe form of this disease, which can cause serious eye disease, fatal encephalitis in immunosuppressed individuals, and miscarriage or birth defects in pregnancy. The prevalence of this parasite alludes to it as one of the most harmful zoonotic diseases in the world, which is explained by its neurotropic nature and high morbidity and mortality rates in immunocompromised patients and newborns [102].
De Assis et al. [103] tested the anti-toxoplasma activity of peptides derived from the venom of the yellow scorpion Tityus serrulatus. As a result, this study observed that such peptides reduced the replication of tachyzoites in macrophages showing no cytotoxicity. Mice infected with T. gondii were treated using the aforementioned peptides, showing a decrease in the number of brain cysts while inducing no hepatotoxicity in the animals tested. Hence, the authors conclude that the data present promising immunomodulatory and antiparasitic activities of these peptides, which could be explored and applied in future therapies to treat toxoplasmosis.
In a similar approach, Liu et al. [104] tested a peptide (XYP1) derived from the venom gland of the spider Lycosa coelestis. This peptide exhibited potent anti-toxoplasma activity in vitro and in vivo. XYP1 treatment significantly inhibited the viability, invasion, and proliferation of tachyzoites in human host cells, with no cytotoxicity and increased the survival rate of mice acutely infected with T. gondii. Notably, the mechanism of action of XYP1 may be related to tachyzoite membrane disruption. In conclusion, the authors propose the possibility of XYP1 being a promising new drug candidate for the treatment of toxoplasmosis.
Hookworm is an intestinal parasite that infects nearly 230 million people, with another 5.1 billion at risk, especially in poverty-stricken tropical and subtropical regions [105]. Chronic hookworm infection can induce iron deficiency anemia, with children and women of childbearing age being the most vulnerable. Currently, control efforts rely on the mass administration of drugs that treat established infections but do not prevent reinfection [106].
In search of new anthelmintics, SAMPs hold great promise, especially the cyclotide family, plant cyclic peptides with approximately 30 aa [41], which have already been presented with anthelmintic activity [107]. Colgrave et al. [108] demonstrated the in vitro anthelmintic activity of three cyclotides—kalata B1, kalata B6, and cycloviolacin O14—on the viability of the larval and adult stages of the canine hookworm Ancylostoma caninum and only the larval stage of the human hookworm Necator americanus. The authors conclude by reinforcing the promising activity of cyclotides as novel anthelmintics.
Human taeniasis is an infectious disease caused by the ingesting of the larval stage of metacestode, the cysticerci of Taenia saginata in beef or Taenia solium in pork, which draws great attention for its ability to cause neurocysticercosis, one of the main causes of neurological system morbidity worldwide [109]. The taeniasis/cysticercosis complex is included in the list of neglected tropical zoonoses of the World Health Organization and that of the Food and Agriculture Organization [110]. This disease is more prevalent in the regions of Asia, Africa, and Latin America, where the disease remains endemic [111].
Landa et al. [112] tested the ability of two AMPs, temporin A (TA, from frog Rana temporaria) and Iseganan IB-367 (IB-367, a synthetic analog of porcine protegrin) to damage T. crassiceps cysticerci in vitro. These peptides caused cysticerci shrinkage, loss of motility, formation of macrovesicles in the tegument, and a decrease in evagination properties. In addition, peptides administered to cysticercotic mice 1 month after infection in a single intraperitoneal dose reduced the parasite load by 25% to 50%. The authors suggest that these findings may contribute to the design of new drugs to prevent and treat such diseases.
Leishmaniasis is a zoonotic disease caused by sand flies infected with an obligate intracellular protozoan parasite (family Trypanosomatidae), which presents itself in three different forms: cutaneous, mucocutaneous, and visceral [113]. The clinical manifestation of this disease varies according to the parasite species and ranges from physical disfigurement to death if left untreated. Despite being first discovered in India, the parasite has been located in several other countries around the world as well. As of 2018, according to the World Health Organization, 94% of the total new cases occurred in seven countries: Brazil, India, Kenya, Somalia, South Sudan, Ethiopia, and Sudan. The disease is endemic in 88 countries, 72 classified as developing countries [114].
Peptide-based drugs are currently being used to develop innovative therapies for various health conditions, including tropical diseases, such as leishmaniasis [115].
In this context, Kumar et al. [116] tested tachyplesin peptide (from the Japanese horseshoe crab Tachypleus tridentatus) against Leishmania donovani. This peptide was established to be active against both forms of the parasite, showing no toxicity to host cells at the concentration used while exhibiting a mode of action that destabilizes the membrane of the protozoan, thus hindering the development of resistance of this parasite. The authors conclude by emphasizing the importance of further analyzing the peptide/parasite interaction and stating that the anti-leishmanial property of tachyplesin makes it appealing as a future drug in leishmaniasis treatment.
Cao et al. [117] examined a peptide of only four amino acids (KDEL, based on the Pseudomonas aeruginosa exotoxin PE) against the promastigote and amastigote of Leishmania tarentolae. The results illustrated the dose-dependent activity of the peptide against this protozoan. In addition, it was possible to observe that the mode of action of this peptide was directly linked to the ability to disrupt the integrity of the parasite’s surface membrane and thus cause cellular apoptosis. Despite having only four amino acids, KDEL has shown therapeutic potential as a new antileishmanial drug.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10081591
This entry is offline, you can click here to edit this entry!
