Multiple sclerosis (MS) is an autoimmune-mediated degenerative disease of the central nervous system (CNS) characterized by immune cell infiltration, demyelination and axonal injury. Oxidative stress-induced inflammatory response, especially the destructive effect of immune cell-derived free radicals on neurons and oligodendrocytes, is crucial in the onset and progression of MS. Therefore, targeting oxidative stress-related processes may be a promising preventive and therapeutic strategy for MS. Animal models, especially rodent models, can be used to explore the in vivo molecular mechanisms of MS considering their similarity to the pathological processes and clinical signs of MS in humans and the significant oxidative damage observed within their CNS. Consequently, these models have been used widely in pre-clinical studies of oxidative stress in MS. To date, many natural products have been shown to exert antioxidant effects to attenuate the CNS damage in animal models of MS. This review summarized several common rodent models of MS and their association with oxidative stress. In addition, this review provides a comprehensive and concise overview of previously reported natural antioxidant products in inhibiting the progression of MS
- multiple sclerosis
- animal model
- oxidative stress
- natural products
- antioxidants
1. Introduction
Multiple sclerosis (MS) is a classical autoimmune disease of the central nervous system (CNS) [1] that occurs mainly in young adults, with a prevalence rate of approximately 2 to 150 cases per 100,000 people [2]. Currently, there are approximately 2.8 million people worldwide with MS [3], with a male to female ratio of 1:2–1:3 [4]. The majority of patients initially present with the relapsing-remitting type of the disease, which evolves to a secondary progressive type within 5–15 years [5]. MS is associated with a high disability rate and has a significant negative impact on the health and quality of life of patients. The aetiology and pathogenesis of MS are unclear and may be associated with genetic, environmental, viral and immune factors [6]. The main pathological features of MS include inflammatory response, demyelination and axonal and neuronal injury [7]. Immunoinflammation-induced demyelination is the main cause of severe neurological dysfunction in patients with MS. Due to the spatial and temporal multiplicity of the lesions and the disease course [8], respectively, the clinical symptoms of MS are complex and recurrent. The primary symptoms of MS include blurred vision, motor abnormalities such as limb weakness and paralysis, sensory abnormalities such as numbness, tingling sensation and zonesthesia, as well as urinary and bowel disorders and memory loss that worsen progressively [9,10]. Inadequate remyelination at the site of injury results in neurodegenerative lesions leading to chronic disability [11]. Furthermore, 80% of the patients experience a relapsing-remitting course, eventually progressing to paralysis or blindness [12]. The prevalence of MS has been increasing worldwide over the past decades; however, there are still some limitations that restrain the efficacy and safety of MS treatment. Glucocorticoids, interferons and immunomodulators are the main modern medical agents that were used in the treatment of MS [13]; however, the associated adverse effects hinder their large-scale application [14]. Despite the indefinite aetiology, the pathological characteristics of MS have been clearly described and chiefly comprise inflammatory cell (peripheral origin) infiltration, oligodendrocyte damage, destruction of neuronal axons and activation of resident immune cells (RICs) in the CNS [15]. Such changes have been observed within the white and grey matter regions of the brain and the spinal cord. Moreover, recent studies have reported that the rapid response of the RICs within the CNS, especially the activation of microglia [16] and astrocytes [17], is associated with the expression and release of oxidative stress-related molecules [18,19], which have a significant effect on chronic myelin loss and impede remyelination and repair [20].
The oxidative stress on the immune and neuronal cells in MS was generalizedin this review. In addition, several commonly used rodent models of MS were studied, and their relationship with oxidative damage was summarized. Furthermore, we highlighted the potential antioxidant and anti-demyelinating effects of natural compounds of plant origin. Figure 1 summarises the mechanisms of oxidative stress generation in the CNS of animal models of MS and the neuroprotective effects of natural antioxidants.
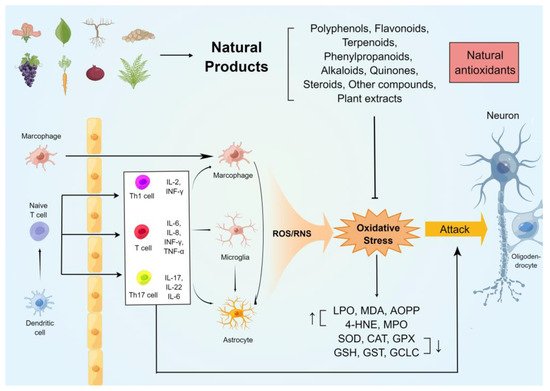
2. Natural Compounds against Oxidative Stress in Animal Models of MS
The antioxidant effects of natural products in MS have been extensively studied in recent years. Natural products have been used in rodent models of MS and have been found to reduce CNS and peripheral immune-inflammatory responses, alleviate demyelination and axonal degeneration, and attenuate oxidative stress damage in the brain, spinal cord and optic nerve, thereby ameliorating various clinical symptoms of MS. The mechanisms of antioxidant and neuroprotective effects of these natural products in animal models of MS (rats and mice) are summarized in Table 1.
| Compound | Experimental Model | Treatment Time Point and Path | Dosage | Antioxidative Stress Effect | Major Results |
|---|---|---|---|---|---|
| Anthocyanins | EB (male Wistar rats) |
Starting from EB injection day (oral) |
30, 100 mg/kg for 7 days | ↑ HNE-His、MDA、NOX ↑ NPSH、GSH、SOD |
↓ IL-6、IL-1β、TNF-α、IFN-γ. ↑ IL-10. ↑ Na +、K + -ATPase、Ca2+-ATPase. |
| Hesperidin | EAE (female C57BL/6 mice) |
After 14th day induction of EAE (i.p. injection) |
100 mg/kg/d for 7 days | ↓ MDA. ↑ GPx、SOD、CAT、GSH |
↓ Caspase-3、IL-17、TNF-α and IL-1β. ↓ inflammatory infiltration。 |
| Licochalcone A | EAE (female C57BL/6 mice) |
Starting at 10th days post-immunization (oral) |
15, 30 mg/kg/d for 10 days | ↓ H2O2, NO | ↓ TNF-α、IFN-γ and IL-17. ↓ Th1 and Th17 cells。 |
| Flavocoxid | EAE (female C57BL/6 mice) |
Prevention protocol: Starting from immunization day therapeutic treatment protocol: from day 14 post immunization (i.p. injection) |
100 mg/kg/d every other day Prevention protocol: for 28 days. Therapeutic treatment protocol: for 19 days. |
↓ iNOS、COX2、5-LOX | ↓ IL-6、IL-12、IL-23、IL-17、IFN-γ. ↓ Th1/Th17 cells. ↑ Arg1、Ym1、CD206、TGF-β、IL-10. |
| Luteolin | EAE (female Wistar rats) |
Starting at 10th day post-immunization (i.p. injection) |
10 mg/kg/d,for 33 days | ↑ TAC | ↑ CNTF,cAMP。 ↓ c-caspase-3、NF-κB、MIP-1α. |
| Epimedium flavonoids | EAE (female Lewis rats) |
Starting from immunization day (oral) |
20, 60 mg/kg/d,for 15 days | ↓ NO,iNOS | ↓ IL-1β、TNF-α、IκB-α、Iba1、CD3、GFAP. ↑ CNPase,NGF. |
| Kolaviron | CPZ (male Wistar rats) |
Starting from cuprizone diet fed (oral) |
200 mg/kg/d both cuprizone and kolaviron for 42 days |
↓ MDA. ↑ GPx, SOD |
↑ the neuronal integrity. |
| Quercetin | EB (male Wistar rats) |
Starting from EB injection day (i.p. injection) |
50 mg/kg/d for 22 days |
↓ MDA. ↑ CAT, SOD. |
↓ AChE. ↓ Na+, K+-ATPase. |
| Phloretin | EAE (C57BL/6J mice) |
Starting after 6 days of immunization or after disease onset (i.p. injection) |
50 mg/kg/d for 10 days or 20 days |
↓ ROS, NO, NOS2, ↑ Nrf2, NQO1 |
↓ MHC-II, CD86, TNF-α, IL-6, CCL4, CCL5, CXCL2. ↑ IL-4, CNTF, IGF-1, p-AMK, P62, LC3-II. |
| Curcumin | EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
20 mg/kg/d for 21 days |
↑ GPx, SOD. | ↓ IL-6, IL-17, TNF-α, IFN-γ. ↑ TGF-β. |
| EAE (female Lewis rats) |
Starting at 12th day post-immunization | 12.5 mg/kg/d for 18 days |
↓ iNOS, ↑ HO-1, Nrf2 | ↓ IL-1, IL-17. ↑ IL-4, IL-10, TGF-β, BDNF, NGF, MBP, Nestin. |
|
| Resveratrol | EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
10, 25, 50 mg/kg/d for 20 days |
↓ iNOS, NOX2, NOX4. ↓ NADPH activity |
↑ Arg1 and IL-10 ↑ ZO-1, Occludin, claudin-5, ICAM-1, VCAM-1. ↑ the BBB integrity |
| CPZ (male C57BL/6 mice) |
cuprizone diet for 7 days, followed by 3 weeks on 0.2 % cuprizone diet plus resveratrol (oral) |
250 mg/kg/d for 21 days |
↓ LPO. ↑ GSH, SOD, cytochrome oxidase | ↓ Rel-A, pIκB-α, TNF-α. ↑ MBP, CNP, Olig1. |
|
| Acteoside | EAE (female C57BL/6N mice) |
Prevention protocol: Starting from day 2 post immunization therapeutic treatment protocol: from day 11 post immunization (oral) |
Prevention protocol: 30 mg/kg/d for 29 days therapeutic treatment protocol: 5, 10 and 30 mg/kg/d for 19 days |
↓ ONOO-, iNOS, NADPH oxidases. | ↓ LC3-II to LC3-I in mitochondrial fraction. ↓ the translocation of Drp1 to the mitochondria. ↓ neuronal apoptotic. |
| Oleacein | EAE (female C57BL/6J mice) |
Starting from immunization day (i.p. injection) |
10 mg/kg/d for 24 days |
↓ O2−, MDA, AOPP, ROS, iNOS, COX2. ↑ FRAP, Sestrin-3 |
↓ TNFα, IL-13, IL-33, IL-1β, MCP-1. ↑ IL-10. ↑ the BBB integrity. ↓ inflammatory infiltration. |
| Ellagic acid | CPZ (male C57BL/6 mice) |
Starting from cuprizone diet fed (oral) |
5, 50, 100 mg/kg/d coadministration of CPZ and Ellagic acid for 6 weeks |
↓ MDA, ROS. ↑ CAT, SOD. |
↑ the integrity of myelin in spinal cord and sciatica. |
| Arbutin | LPC (male Wistar rats) |
Starting from LPC injection day (i.p. injection) |
50 mg/kg/d for 14 days |
↑ Nrf2, HO-1. | ↓ IL-1β, IL-17, TNF-α, GFAP. ↑ IL-10, MBP, Olig2. |
| Paeonol | CPZ (male C57BL/6 mice) |
Started 1 week after beginning cuprizone challenge till the end of week 6 post-cuprizone (oral) |
25 mg, 100 mg/kg/d for 42 days |
↓ MDA, ROS, MPO. ↑ CAT, SOD, GSH. |
↓ NF-κB, TNF-α. ↑ MBP. |
| 18β-glycyrrhetinic acid | EAE (female C57BL/6 mice) |
14 days after induction of EAE (i.p. injection) |
100 mg/kg/d for 7 days |
↓ MDA. ↑ GPx, SOD, CAT, GSH | ↓ Caspase-3, IL-17, TNF-α, IL-1β. |
| β-Caryophyllene | EAE (female C57BL/6 mice) |
Starting at 10th day post-immunization (oral) |
25, 50mg/kg/d for 9 days |
↓ H2O2, NO | ↓ TNF-α, IFN-γ, IL-17 |
| Ginkgolide K | CPZ (male C57BL/6 mice) |
Starting from 5th weeks until the end of 6th weeks (oral) |
20 mg/kg/d for 14 days |
↑ Nrf2, HO-1. ↓ NO, iNOS. |
↓ IL-1β, IL-6, TNF-α. ↓ p-NF-kB/p65, caspase-3, TUNEL. ↑ IGF/PI3K. |
| Bixin | EAE (female C57BL/6 mice) |
Starting at 12th days post-immunization (i.p. injection) |
50, 100, 200 mg/kg/d for 18 days | ↓ ROS, Nrf2, TXNIP, NLRP3 | ↓ TNF-α, IL-6, IL-8, IL-17, and IFN-γ. ↑ IL-10. ↓ Th1 and Th17 cells. |
| Acetyl-11-keto-β-boswellic acid | EAE (female SJL/J mice) |
starting at day 11 after immunization (oral) |
20 mg/kg/d for 30 days |
↑ Nrf2, HO-1, TAC ↓ iNOS, lipid peroxides |
↓ p-NF-κB, NF-κB, IL-6 |
| Ginsenoside-Rg3 | EAE (female C57BL/6N mice) |
Starting from 7 days before immunization (oral) |
37.5, 75, 150mg/kg/d for 28 day |
↓ MitoSOX, 4-HNE, NOX2, NOX4, NADPH activity, COX2, iNOS | ↑ occludens-1, claudin-3, and claudin-5. ↓ TNF-α, IL-6, IL-1β. |
| Astragaloside | EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
10, 25, 50 mg/kg/d for 15 days |
↓ ROS, MDA, iNOS ↑ GPx, SOD |
↓ p53, p-tau ↑ Bcl-2/Bax ratio. ↑ the BBB integrity. |
| Nordihydroguaiaretic acid | EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
10 mg/kg/d for 30 days |
↓ MDA. ↑ HO-1 | ↓ IL-17 ↓ p38MAPK, SGK1. ↓ inflammatory infiltration. |
| Caffeic acid phenethyl ester | EAE (female Wistar rats) |
Starting from the first day of immunization (i.p. injection) |
25 μmol/kg/d for 14 days |
↑ GPx, ADA, SOD. ↓ MDA, NO, XO. |
↓ inflammatory infiltration. |
| P-Coumaric Acid | CPZ (female and male C57BL/6 mice) |
Starting from 7th weeks until the end of 12th weeks (oral) |
21.6 ppm/mouse/d for 42 days |
↓ free radical (DPPH) | ↓ MMP-9 |
| Magnolol | EAE (female Swiss mice) |
Starting from immunization day (oral) |
0.1, 1, 10 mg/kg/d for 21 days |
↓ MDA, MPO, NO, iNOS. ↑ GSH, GST, SOD, Nrf2. | ↓ CD8+ T cell. ↓ c-caspase-3. |
| Daphnetin | EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
8 mg/kg/d for 21 days |
↓MDA, HO-1 | ↓ IFN-γ, IL-17, IL-1β, IL-6, TNF-α. ↑ IL-10. inflammatory infiltration. |
| Piperine | EAE (female Lewis rats) |
Starting at 8th day post-immunization (i.p. injection) |
5 mg/kg/d for 22 days |
↓ iNOS, MDA. ↑ Nrf2, HO-1 FRAP. |
↓ TNF-, IL-1β, caspase-3. ↑ IL-10, BDNF, MBP. |
| LPC (Male Wistar rats) |
Starting at 3 days post LPC injection (i.p. injection) |
5, 10, 20 mg/kg/d for 10 days |
↑ Nrf2, HO1, FRAP. ↓ iNOS. |
↓ TNF-α, IL-1β, NF-κB, Foxp3. ↑ IL-10, BDNF, MBP. |
|
| Matrine | EAE (Female Wistar rats) |
starting from day 11 post immunization (i.p. injection) |
250 mg/kg/d for 7 days |
↓ MDA. ↑ GPx | ↓ caspase-3, α-B-crystallin, Cyt. ↑ Beclin1, LC3. |
| Shikonin | EAE (female C57BL/6 mice) |
After EAE was induced (i.p. injection) |
20 mg/kg/d for 21 days |
↑ GPx1 | ↓ TNF-α, IFN-γ and Bax. ↑ TGF-β and Bcl2. |
| Thymoquinone | EAE (female Lewis rats) |
at days 1–5 post immunization or at day 12–17 post immunization (i.v. injection) |
1 mg/kg for 5 days or 6 days |
↑ GSH | ↓ IL-7, IL-7R ↓ inflammatory infiltration. |
| Withametelin | EAE (Female Swiss mice) |
starting at day 9 through day 25 (i.p. injection) |
10, 100, and 1000 μg/kg/d for 17 days |
↓ NO, MPO, iNOS ↑ GSH, SOD, Nrf2, HO-1 |
↓ TLR4, NF-κB, AP-1. ↑ IκB-α, Bcl-2. |
| Guggulsterone | EB (Wistar rats) |
Starting at 8th day post EB injection (oral) |
30, 60 mg/kg/d for 28 days |
↓ AchE, MDA, NO. ↑ SOD, CAT, GSH. |
↓ IL-1β, TNF-α. ↓ caspase-3, Bax, STAT3. ↑ Bcl2, PPAR-ϒ, MBP. |
| Sulforaphane | EAE (female C57BL/6N mice) |
14 days before EAE induction (oral) |
50 mg/kg/d for 28 days |
↓ iNOS | ↓ CD4, CD68, GFAP ↓ inflammatory infiltration. |
| EAE (female C57BL/6 mice) |
Starting from immunization day (i.p. injection) |
50 mg/kg every other day up to 22 days |
↓ HO-1, NQO1 ↑ Nrf2 |
↑ Occludin, claudin-5. ↑IL-10. ↓ MMP9, Th17 cells. |
|
| 3H-1,2-dithiole-3-thione | EAE (female C57BL/6N mice) |
starting at day 1 post immunization (i.p. injection) |
10 mg/kg/d for 30 days |
↑ Nrf2, NQO1, GCLC, HO-1. ↓ iNOS |
↓ IL-23. ↓ Th1 and Th17 differentiation. |
| C-Phycocyanin | EAE (female C57BL/6 mice) |
Treatments started at disease onset (i.p. injection) | 2, 4, 8 mg/kg/d for 15 days |
↓ MDA, peroxidation potential, CAT/SOD ratio. ↑ GSH. | ↓ CD3, Mac-3 ↓ IL-17, IL-6, Foxp3 ↓ inflammatory infiltration |
| Artemisia dracunculus L. | EAE (female C57BL/6 mice) |
Starting at 11th day post immunization (oral) |
500 mg/kg/d for 23 days |
↑ TAC (FRAP) |
↓ IL-17, IL-23. ↑ TGF-β. ↓ inflammatory infiltration |
| Olive leaf | EAE (female C57BL/6 mice) |
starting from the first day after EAE induction. (olive leaf tea, oral) starting from the 8th day after EAE induction (olive leaf extract, i.p. injection) |
Olive leaf tea: ad libitum for 20 or 30 days Olive leaf extract: 1024 mg/kg/d for 10 days |
↓ MDA ↑ SOD1, SOD2, GPx1 |
↑ SIRT. ↓ M1 microglia. ↑ M2 microglia. |
| Melilotus officinalis | EAE (female C57BL/6 mice) |
starting from first day post-immunization (i.p. injection) |
10 mg/kg/d for 21 days |
↑ CAT, GPx1 | ↓ IL-6, TNF-α, IFN-γ, IL-17. ↑ TGF-β, IL-5. |
| Nutshell of Xanthoceras sorbifolia | EAE (female C57BL/6 mice) |
Starting from 5 days before immunization (oral) |
50, 100, 150 mg/kg/d for 35 days | ↓ free radical (DPPH) | ↓ Th1, Th17 cells. ↓ p-STAT1, p-STAT3, p-STAT4. ↓ IL-17, IL-1α, IL-1β, TNF-α, CCL1, CCL2, CXCL1, CXCL10, CXCL11. |
| Crocus sativus L. | EAE (male C57BL/6 mice) |
Starting from immunization day (oral) |
500 mg/kg/d for 21 days |
↑ TAC (FRAP). ↓ NO |
↓ inflammatory infiltration |
| Moringa oleifera. | CPZ (Wistar rats) |
Starting from cuprizone diet fed (oral) | 1.875 mg/mL/mouse/d for 35 days |
↓ NO ↑ CAT, SOD |
↑ neuronal integrity. |
| Olive oil | EAE (Dark Agouti rats) |
starting at 11th day post immunization (oral) |
representing 10% of calorie intake in the total standard diet for 54 days | ↑ GPx. ↓ LPO, NO. |
↓ NF-κBp65, TNF-α. ↓ LPS, LBP. |
| Copaiba oil | EAE (female C57BL/6 mice) |
Splenocytes were obtained from EAE mice at day 20 post immunization (in vitro) |
100, 50 and 25 µg/mL for 24 h |
↓ H2O2, NO. | ↓ TNF-α, INF-γ, IL-17 |
| Sesame oil | EAE (male C57BL/6 mice) |
Starting from day 3 before the immunization (oral) |
4 mL/kg/d for 28 days |
↓ NO ↑ TAC (FRAP). |
↓ inflammatory infiltration. |
| Hypericum perforatum L. extract |
EAE (C57BL/6 mice) |
Starting from immunization day (oral) |
18−21 g/kg/d for 42 days |
↑ TSA. ↓ TOS, OSI. |
↑ MOG, MBP. ↓ inflammatory infiltration. |
| Oenothera biennis L. extract |
EAE (C57BL/6 mice) |
Starting from immunization day (oral) |
18−21 g/kg/d for 42 days |
↑ TSA. ↓ TOS, OSI. |
↑ MOG, MBP. ↓ inflammatory infiltration. |
2.1. Natural Phenolic Compounds
2.1.1. Flavonoids
2.2. Terpenoids
2.3. Phenylpropanoids
2.4. Alkaloids
4.5. Quinones
Shikonin is a class of natural naphthoquinones extracted from the roots of Lithospermum erythrorhizon. Shikonin treatment of EAE mice significantly reduced the extent of corpus callosum demyelination. The level of ROS in MS increased, and the antioxidant defense system in vivo was damaged, which led to the enhanced blood–brain barrier permeability. Various inflammatory factors enter the brain and spinal cord in large quantities. Among them, TNF-α directly induces oligodendrocyte death and oligodendrocyte progenitor cell loss. IFN-γ can promote the inflammatory response of EAE and MS. The accumulation of these cytokines significantly increased the expression level of apoptosis-related proteins in CNS. Conversely, TGF-β is an anti-inflammatory cytokine, mainly produced by T cells, monocytes, astrocytes, and microglia, which can prevent autoimmune response and inflammatory damage. The genes expression of TNF-α, IFN-γ and Bax was enhanced and TGF-β and Bcl2 were reduced in the brain tissue of EAE mice, and shikonin treatment significantly reduced the expression levels of TNF-α, IFN-γ and Bax. In addition, the expression levels of TGF-β and Bcl2 and the activity of GPx1 were significantly increased after shikonin treatment. These results suggest that shikonin has good immunomodulatory and antioxidant effects in EAE and may contribute to the remission of EAE [186].
Thymoquinone (TQ), a benzoquinone, is a natural antioxidant isolated from the seeds of Nigella sativa and present in other plants [187]. Increased IL-17 and IL-17R have been reported in an EAE-induced demyelination mouse model, and treatment with TQ reduced this abnormal expression. In addition, TQ inhibited the development of acute and chronic recurrent EAE in mice, reduced the number of perivascular inflammatory cell infiltrates and increased GSH in the spinal cord, suggesting that TQ may reduce myelin damage in EAE mice by inhibiting oxidative stress [188,189].
4.6. Steroids
Withametelin (WMT), a natural sterol lactone derived from the leaves of Datura stramonium, has been shown to relieve depression and neuropathic pain significantly [190]. Dysfunction of the blood–brain barrier (BBB) plays a critical role in the pathogenesis of MS, with the migration of pro-inflammatory cells and toxic molecules to the brain through the damaged BBB, leading to demyelination and neuronal death. Fourier transform infrared spectroscopy revealed that EAE induced significant changes in myelin biomolecular composition, including protein oxidative damage, lipid peroxidation, increased nucleic acid/carbonyl content, and decreased lipid/protein content. It is well known that Nrf2/Keap-1-mediated oxidative stress and neuroinflammation contribute to neuronal degeneration in EAE models of MS. Oxidative stress in the brain, spinal cord, and optic nerve may cause permanent cellular damage due to the oxidation of cellular components. In MS, low levels of antioxidant enzymes and high levels of reactive oxygen species aggravate CNS damage. WMT treatment significantly attenuated EAE-induced weight loss, neuropathic pain, and motor dysfunction reduced elevated circulating leukocytes and blood–brain barrier disruption, and reversed histopathological changes in the brain, spinal cord, and optic nerve. WMT enhanced the antioxidant defense mechanism and decreased the expression of Keap-1 and iNOS by increasing the expression levels of Nrf2 and HO-1 in the CNS [191].
Guggulsterone (GST) is a natural phytosterol from the resin of Commiphora mukul, which is widely used in preclinical animal studies of CNS diseases such as ischemic stroke, dementia, depression, and autism [192]. PPAR-γ plays an important role in neuroinflammation, and up-regulation of PPAR-γ can reduce pathological expression in EAE models. Elevated PPAR-γ enhances remyelination in MS by suppressing T cells. It has been reported that PPAR-γ agonists reduce ROS production, protect mitochondria, and promote oligodendrocyte differentiation and maturation. Conversely, inhibition of JAK/STAT-mediated glial activation was neuroprotective, reduced interleukin and Th1 cell differentiation, and attenuated oxidative damage. GST improves behavioral deficits (spatial cognitive memory, grip and motor coordination) and increases the expression of the myelin marker MBP in EB demyelinated rats. GST also modulates neurotransmitter levels by increasing acetylcholine, dopamine, serotonin and decreasing glutamate. In addition, GST ameliorates inflammatory cytokines (TNF-α, IL-1β) and oxidative stress markers (AchE, SOD, CAT, MDA, GSH, NO) to prevent EB-induced apoptosis. These effects were associated with the dowregulation of JAK/STAT and upregulation of PPAR-γ signaling pathways by GST [193].
4.7. Other Compounds
Sulforaphane (SFN) is an organosulfur compound derived from cruciferous vegetables (e.g., cauliflower). SFN exerts neuroprotective effects through its antioxidant effect in CNS diseases such as Alzheimer’s disease, Parkinson’s disease, epilepsy, etc. It is reported that ROS reduces the integrity of BBB and facilitates the entry of peripheral immune cells into the CNS. Infiltrated leukocytes produce ROS and induce oligodendrocyte and axon damage. In addition, reactive microglia produce peroxynitrite, the main mediator of oxidative stress and neuronal excitotoxicity, thus driving the neurodegenerative process in MS. Nrf2 is a redox-sensitive transcription factor. Previous studies have shown that Nrf2/ARE transcription pathway is crucial for cell defense against oxidative damage. SFN treatment inhibits inflammatory infiltration, demyelination and upregulation of iNOS and NO in the spinal cord of EAE mice. Another study showed that SFN protected the blood–brain barrier in EAE mice and by upregulating Nrf2/ARE pathway to activate the antioxidant HO-1 and NADPH quinone oxidoreductase 1 (NQO1) expression levels. In addition, SFN treatment can inhibit Th17 response and enhance the release of IL-10. These results suggest that SFN inhibits the development of EAE in mice through its antioxidant and anti-autoimmune inflammatory activities [194,195].
3H-1,2-dithiole-3-thione (D3T) is a compound containing a five-membered cyclic sulfur structure extracted from cruciferous vegetables. D3T effectively induces activation of cellular antioxidant and anti-inflammatory defense systems and provides protection in a variety of disease models. In MS/EAE, antigen-presenting cells activate naive T cells and produce inflammatory cytokines to promote the differentiation of encephalitogenic CD4+ T cells. Dendritic Cells (DCs) have been shown to play a key role in promoting the development of pathogenic Th1/Th17 cells. The proinflammatory cytokines IL-12 and IL-23 produced by DCs have been shown to be critical for Th1 and Th17 differentiation, respectively. The administration of D3T after the onset of EAE effectively prevents disease progression. Pharmacological studies have shown that D3T inhibits dendritic cell activation, suppresses the differentiation of Th1 and Th17, and inhibits microglia activation and inflammatory cytokine expression. In vitro experiments revealed that D3T strongly induced Nrf2 and HO-1 expression and enhanced antioxidant activity in LPS-stimulated dendritic cells [196].
C-Phycocyanin (C-Pc) is a photosynthetic pigment isolated from Spirulina platensis with significant effects in regulating excessive oxidative stress, inflammatory damage and immune responses. There was a massive inflammatory infiltration consisting of lymphocytes and macrophages/activated microglia in animals with EAE. Microglia comprise about 10% of all brain cells and are the first line of CNS immune defense. Most studies have shown that activated microglia exacerbates MS/EAE pathogenesis by producing neurotoxic molecules, proinflammatory cytokines, and oxygen-free radicals. Furthermore, IL-17 and IL-6 are the primary effector cytokines in MS, and their knockout mice are protected from EAE. Therefore, it is necessary to intervene in immune cell-mediated inflammatory responses and oxidative damage. C-Pc can ameliorate clinical deterioration, reduce Inflammatory macrophage/microglia infiltration in spinal cord tissue and IL-6, IL-17 in the brain and serum, and regulates oxidative stress parameters (lower MDA, higher GSH) in the peripheral blood of EAE mice, thereby slowing oxidative damage and increasing myelin repair and regeneration in the CNS [197].
4.8. Plant Extracts
Artemisia dracunculus L. is a perennial herb of the Asteraceae family and is an important herb in traditional medicine in many countries. Modern pharmacological studies have demonstrated that it has inhibitory effects on inflammation and oxidative damage, and hepatoprotective and neuroprotective effects [198]. In MS/EAE, Th17 cells can produce IL-17 and IL-23. IL-17 can inhibit the differentiation and maturation of oligodendrocytes. Moreover, IL-17 can enhance the apoptosis-induced effect of TNF-α in oligodendrocytes, which is mainly related to mitochondrial dysfunction, ROS generation, and cell cycle arrest. Administration of A. dracunculus aqueous extract alleviated weight loss, inflammatory infiltration and demyelination of CNS in EAE mice. After treatment, serum levels of inflammatory cytokines, including IL-17 and IL-23, were reduced, while antioxidant levels (FRAP) were increased [199].
Olive leaves are widely used as a medicinal plant in the Mediterranean region. Some evidence suggests that it contains high levels of phenolics with antioxidant effects to prevent and treat neurodegenerative diseases. The reduction of SIRT1 exacerbates the progression of various neurodegenerative diseases, including MS. Inhibition of SIRT1 expression may contribute to microglial activation and neuroinflammation. SIRT1 has been shown to affect the redox properties of cells and reduce oxidative stress by regulating FOXO3a, resulting in increased activity of CAT and SOD. Based on these facts, SIRT1 is a promising target for MS therapy. Oral administration of olive leaf tea combined with olive leaf extract intraperitoneally in EAE mice attenuated the severity of MS and suppression of SIRT1, upregulated antioxidant enzymes (SOD1, SOD2 and GPx1) and M2 microglia, and inhibited M1 phenotype to maintain myelin integrity [200].
Melilotus Officinalis is an herb from traditional medicine, mainly distributed in East Asia, the Middle East and the eastern shores of Mediterranean, with good anti-inflammatory effects [201]. Disruption of Th1/Th2 balance and oxidative damage and apoptosis of oligodendrocytes play a key role in the pathogenesis of MS. IL-6, TNF-α, IL-12, and IFN-γ are secreted by Th1 cells, but Th2 cells release anti-inflammatory cytokines, such as IL-4, and IL-5, which are vital factors affecting axonal and myelin damage. Prophylactic administration of Melilotus Officinalis extracts attenuated clinical symptoms and pro-inflammatory factors such as IL-6, TNF-α and IFN-γ in the corpus callosum of EAE mice. This herbal extract also promoted the expression of anti-inflammatory cytokines and antioxidant enzymes (CAT, GPx1), thereby maintaining the structural integrity of myelin[202].
Xanthoceras sorbifolia Bunge has long been used in China as traditional folk medicine [203]. Its fruit shell extract (NE) contained high natural phenolic compounds and potent antioxidants. In vivo results showed that oral administration of NE effectively improved clinical disease severity and reduced CNS demyelination in EAE mice. The phosphorylation of STAT1 and STAT3 was highly expressed in CNS of EAE mice, which promoted the polarization of Th1/Th17 cells. NE inhibited Th1 and Th17 cell differentiation via modulating JAK/STAT signaling pathway and reduced the entry of brain inflammatory immune cells into the CNS. In addition, NE exhibited extremely strong antioxidant capacity in vitro and reduced the level of DPPH free radicals [204].
Saffron comes from the flowers of Crocus sativus L., a common medicinal plant whose neuroprotective effects in CNS disorders such as depression, anxiety, Alzheimer’s, Parkinson’s, and epilepsy have received much attention in recent years. In vivo studies showed that oral administration of saffron extracts significantly delayed the onset of EAE disease and attenuated clinical symptoms and CNS inflammatory cell infiltration in mice. Oxygen and nitrogen free radicals lead to lipid peroxidation in MS/EAE, exacerbating neuronal and oligodendrocyte damage. Improving antioxidant enzyme activity is beneficial in preventing free radical-mediated CNS inflammatory response and tissue damage. In addition, total antioxidant capacity (TAC) in the serum of EAE mice treated with saffron extracts was significantly increased [205 ]。
Moringa oleifera is a tree that grows widely in many tropical and subtropical countries. The seeds, leaves, flowers and oil of Moringa oleifera are widely used in Southeast Asian traditional medicine. Moringa oleifera has good antioxidant, antidiabetic, antihyperlipidemic, and cardiovascular and neuroprotective effects. CPZ-induced demyelination resulted in memory impairment, increased cortical and hippocampal oxidative stress (inhibited CAT, SOD, increased NO release), and neuronal damage in rats. There is a marked intracellular accumulation of nitrotyrosine-positive protein aggregates in neurodegenerative diseases such as MS. However, administration of Moringa oleifera significantly reversed CPZ-induced neuropathological defects and nitrative stress, while enhancing the antioxidant capacity of rat brain [206]. Olive oil is extracted from the olive fruit and more than 200 compounds, including sterols, carotenoids, triterpene alcohols, and phenols, have been detected in olive oil. The microbiota products (LPS and LBP) in EAE were positively correlated with oxidative stress. LPS and LBP induce activation of the peripheral immune system and increase the permeability of the blood–brain barrier, leading to the persistence of oxidative damage to the CNS. EAE rats treated with olive oil by gavage for 51 days showed a reduction in bacterial LPS and LPS binding protein (LBP) in the brain, spinal cord and blood. LPO and NO, indicators of lipid and protein oxidation, were downregulated and the activity of antioxidant enzymes GSH and GPx was increased, thus slowing down oxidative damage to CNS myelin [207].
Copaiba oil (COP), an oleoresin extracted from genus Copaifera L., is an important phytomedicine in South American traditional medicine with significant bactericidal and anti-inflammatory effects. H2O2 is widely generated in the CNS of MS/EAE, and prolonged exposure to high concentrations of H2O2 can lead to irreversible cell damage. Similar to H2O2, NO is responsible for myelin damage in MS. NO is neurotoxic and is abundantly produced by macrophages and other immune cells. In vitro experiments confirmed that EAE mouse splenocytes were overproduced with oxidative mediators (H2O2, NO) and pro-inflammatory factors (IFN-γ, TNF-α and IL-17) under the stimulation of MOG35–55 and ConA, and oxidative stress and inflammatory responses were significantly inhibited after co-culture with COP for 24 and 48 h [208].
Sesamum indicum, traditional health food in many Asian countries, contains many fat-soluble antioxidants. Daily intraperitoneal administration of sesame oil to EAE mice after immunization effectively delayed the onset of EAE and reduced clinical symptoms. Sesame oil can significantly improve the total antioxidant capacity of serum and inhibit the production of NO. Typical brain inflammatory cell infiltration was observed in EAE mice compared to sesame oil-treated mice. This result suggests that sesame oil effectively prevents disease progression in EAE, which may be related to the inhibition of oxidative stress [209].
Oenothera biennis L. belongs to the Onagraceae family and has long been used in folk medicine as a good natural anti-inflammatory and antioxidant agent [210]. Hypericum perforatum belongs to the Hypericaceae family and its beneficial effects have been demonstrated in treating depression, tumors, and bacterial infections [211]. Recently, the therapeutic effects of O. biennis and H. perforatum on EAE have been reported. Oxygen and nitrogen free radicals produced by macrophages and other immune cells are involved in demyelination and axonal damage in MS. Antioxidants can prevent brain tissue damage caused by free radicals. MS/EAE brain and spinal cord tissue increased TOS and OSI levels and decreased TAS expression. The result showed that extracts of O. biennis and H. perforatum decreased the levels of TOS and OSI, increased TAS levels and reduced clinical symptoms and myelin damage in the brains of EAE mice. EAE mice were also observed to have amyloid deposition in the vessel wall, neuronal cytoplasm and cell interstitial spaces. These abnormal expressions were significantly eliminated in O. biennis and H. perforatum extract-treated groups [212].
This entry is adapted from the peer-reviewed paper 10.3390/antiox11081495
