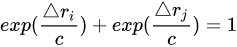The hydrogen bond may be treated as a local stabilizing interaction that acts between the proton or the electron charge deficient region of hydrogen centre and the electron rich region that is related to one or more centres. This may be named as the two-sites hydrogen bond definition. However in numerous studies the A-H proton donating bond with the positively charged H-atom and the proton acceptor, say B, being the electron rich centre are taken into account. Thus so-called A-H∙∙∙B hydrogen bridges are often considered. In such a way the three-sites hydrogen bond definition may be proposed that the hydrogen bond is the A-H∙∙∙B local stabilizing system with the proton situated between two A and B electron rich sites, most often closer to one of them, forming the A-H covalent bond; both A and B may correspond to one or more atomic centres.
- Lewis acid-base interaction
- σ-hole bond
- proton transfer
1. Introduction
It is well known that the hydrogen bond plays a crucial role in numerous chemical, physical and biological processes.[1] There are various controversies connected with the nature of this interaction; one can mention the debates concerning the covalent and electrostatic character of the hydrogen bond.[2]
The debates mentioned above concern even the definition of the hydrogen bond; various definitions were proposed in early and recent studies. One may refer to the early definition of Pauling that "under certain conditions an atom of hydrogen is attracted by rather strong forces to two atoms, instead of only one, so that it may be considered to be acting as a bond between them. This is called the hydrogen bond."[3] Anoher definition introduced by Jeffrey and Saenger may be also mentioned: "A hydrogen bond is therefore the attractive force that arises between the donor covalent pair X-H in which a hydrogen atom H is bound to a more electronegative atom X, and other noncovalently bound nearest neighbor electronegative acceptor atoms A, A´."[4] Two definitions that were proposed very recently are presented at the beginning of this entry (in "the definition window").[5]
The hydrogen bond may be often treated as the preliminary stage of the proton transfer reaction;[2] however it is worth to mention that other Lewis acid - Lewis base interactions are also important in various processes, particularly biochemical ones [6][7] and that they may be followed by chemical reactions.
2. The Hydrogen Bond as a Preliminary Stage of the Proton Transfer Process
It has been described in early studies that a fragment of a crystal structure may be treated as “a frozen stage” of a chemical reaction. The related fragments that differ by geometry and that are taken from various crystal structures may correspond to the analysed chemical reaction since they reflect structural changes accompanying this reaction.[8][9] This approach is known as the structure-correlation method. It was applied to analyse different reactions such as the nucleophilic addition to a carbonyl group, nucleophilic substitution at tetrahedral coordinated atoms (SN1 and SN2 reactions); electrocyclic ring closure of polyenes and other chemical processes.[8]
It is important that the proton transfer, PT, process related to the hydrogen bond, HB, may be also discussed in terms of the structure-correlation method. For example, PT in O-H···O hydrogen bonds was analysed since -C=O···H-O-C- fragments taken from different crystal structures were compared to reconstruct the corresponding reaction path.[2][10] For these analyses, the high-precision neutron diffraction geometries were taken from the Cambridge Structural Database, CSD.[11][12] The recent CSD release (CSD updates up to May 2020) was applied here to search the above-mentioned -C=O···H-O-C- fragment with the following search criteria; accurate crystal structures with e.s.d’s ≤ 0.005 Å, R ≤ 7.5%, error free structures, without disorder, no polymers and no powder diffraction results. Only neutron diffraction results were taken into account here since they are characterised by precisely determined positions of H-atoms [13] in contrast to the X-ray results, where the refinement of crystal structures is usually based on the spherical approximation of the atomic electron densities that results in the spherical symmetry of atomic scattering factors.[14] One may say that the sample of fragments described above and corresponding to different crystal structures of organic and organometallic compounds may reflect the reaction path of the following PT process; -C=O···H-O-C- ⇔ -C-O-H···O=C-. In some of structures H-atom is situated in the mid-point of the O···O distance or near to this point therefore -C=O···H+···O=C- fragments are also included in the sample. The search has led to a finding of 56 geometrical fragments corresponding to the above-mentioned PT process. The similar search with the same criteria for accuracy of results was performed for the similar fragments where the H-atom is replaced by the deuterium, i.e., the -C=O···D-O-C- fragments; in this case only four structures were found.
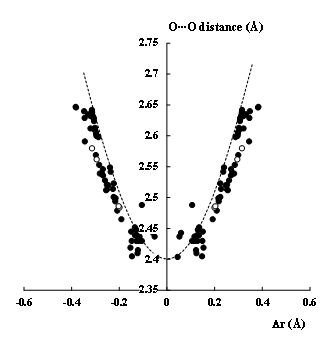
Figure 1 presents the PT reaction path based on two above described CSD searches; this is the relationship between the Δr parameter and the O···O distance. The same relationships were discussed before [2][10] but they were based on earlier CSD updates. The Δr parameter is the distance of the H-atom of the O-H···O bridge from the O···O mid-point. For the linear O-H(D)···O systems the O···O distance may be expressed as the rO-H + rH…O sum while the Δr parameter as the (rH…O − rOH)/2 term. The rOH and rH…O values correspond to the O-H bond length and the H…O distance, respectively. The points of Figure 1, which correspond to fragments of crystal structures, may be considered as positions of the proton in PT process. The results of this figure are symmetrised around Δr = 0; this symmetrisation corresponds to the equivalency of systems during PT reaction because the homonuclear O-H···O hydrogen bond is discussed here. The “points” in the middle of O···O distance may be considered as the transition state of the proton transfer reaction. These are strongly elongated O-H bonds and they are observed for the O···O distances amounting about ~2.4–2.5 Å. For long O···O distances the H-atoms are located far from their mid-points, they are situated close to one of oxygen centres rather.
The broken line of Figure 1 corresponds to the relationship expressing the bond order (number) conservation rule (Equation (1)).[2][8][9]
The Δri and Δrj terms in the above equation correspond to the (r0 − rOH) and (r0 − rH∙∙∙O) differences; r0 is the typical single O-H bond length not perturbed by any interaction. The bond length of water in the gas phase equal to 0.957 Å was chosen here and in other studies.[2] The exponential terms of Equation (1), exp(Δri/c), may be treated as the definition of the bond order. The constant c for the O-H···O hydrogen bonds is determined from the above exponential expression assuming that for the O-H···O linear system with the H-atom located in the O···O mid-point, and the O···O distance of 2.4 Å, two equivalent H···O distances possess the bond order equal to 0.5.
Figure 1. The dependence between the Δr (Å) - the displacement of the proton (or deuter) position from the O···O mid-point and the O···O distance (Å), for the O-H···O systems (black circles) and the O-D···O ones (white circles). The broken line corresponds to the bond number (order) conservation rule (Equation (1)).
The bond order and the bond order conservation rule ideas for the O-H···O hydrogen bonds may be described in the following way. For the single O-H bond in the gas phase, the bond order is equal to unity. If this bond is involved in the hydrogen bond thus it is elongated and its bond order decreases. However, this decrease is compensated by the H···O contact. Hence the sum of bond orders (Equation (1)) of the O-H bond and the H···O contact is equal to unity. The greater is the O-H bond elongation for the stronger hydrogen bond thus the greater is the bond order of the H···O contact that is shorter accordingly; the latter is also accompanied by the decrease of the O···O distance (Figure 1). The extreme cases of very short O···O distances with the H-atom location at the O···O mid-point for very strong hydrogen bonds correspond to the transition states of the proton transfer reaction.
One can see that the broken line of Figure 1 that was derived from the bond order conservation rule is only approximately in agreement with the neutron diffraction results. Figure 1 contains also the O-D···O systems; it is pointed out in several studies that the deuteration of the O-H···O systems results in shortening of the O-D bond and lengthening of the O···O and D···O distances in comparison with their non-deuterated counterparts; it is known as the Ubbelohde effect.[15] Figure 1 shows that the deuterated O-D···O systems are approximately in agreement with the broken curve derived from Equation (1). One may conclude that the reaction path presented in Figure 1 shows that the hydrogen bond, especially for the strong O-H···O interactions, may be treated as the initial stage of PT process.
It is worth mentioning that similar relationships to this one presented in Figure 1 were analysed for other hydrogen bonded systems. For example, it was found that the dependence between q1 and q2 parameters for the N-H···N hydrogen bond geometries taken from experimental NMR and crystal structures´ results is in agreement with the bond order conservation rule expressed by an equation similar to that one presented above here (Equation (1)).[16] The q1 and q2 parameters are equal to (rH…N − rN-H)/2 and rH…N + rN-H, respectively; rH…N is the H···N distance and rN-H is the N-H bond length in the N-H···N system.
The increase of the strength of the hydrogen bond is related to the increase of the probability of occurrence of PT process and the increase of the covalent character of this interaction. The latter may be detected by the Quantum Theory of Atoms in Molecules (QTAIM) approach.[17] The value of the electron density at the H···B bond critical point (BCP), ρBCP, of the order of 0.1 u and more, and the negative Laplacian of this electron density, ∇2ρBCP, inform of the covalent character of the hydrogen bond. However, it is assumed in numerous studies that even for ∇2ρBCP > 0, the negative value of the total electron energy density at BCP, HBCP, shows the partly covalent character of interaction.[2]
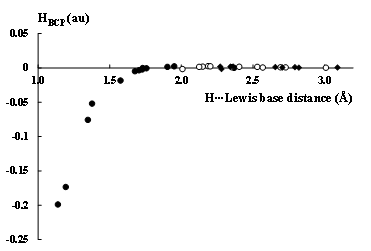
Figure 2 presents the relationship between the hydrogen···Lewis base distance and the HBCP value at the corresponding BCP, for the sample of hydrogen bonds analysed in earlier study.[18] These results are based on the MP2/6-311++G(d,p) calculations. The following complexes linked by hydrogen bonds were discussed there. The complexes connected by charge assisted hydrogen bonds (CAHBs): (FHF)−, H2O···H3O+, H3O+···HCN, OH−···H2O, NH3···NH4+, C5H5−···HF and C5H5−···C2H2. The complexes with π-electrons acting as the acceptor of proton, these are two latter CAHB systems as well as the following species; C2H2···HF, C2H4···HF, C6H6···HF, (C2H2)2 (T-shaped dimer), C2H4···C2H2, C6H6···C2H2, C6H6···CH4, C6H6···CHCl3, C2H2···CH4 and C2H2···CHCl3. There is the sub-sample of complexes linked by the C-H···B hydrogen bonds: F3CH···NCCH3, H3CH···NCCH3, HCCH···NCCH3, F3CH···OCH2, H3CH···OCH2, HCCH···OCH2, H3CH···SH2, HCCH···SH2 and HCCH···S(CH3)2. The other hydrogen bonds analysed in the above-mentioned study may be classified as moderate or strong ones, these are interactions in the following complexes; (C6H5COOH)2, (CH3COOH)2, (HCOOH)2, (HCONH2)2, (HCSNH2)2, (H2O)2 (trans-linear dimer), H2O···HF and H2CO···HF. The hydrogen bonds are divided into three groups in Figure 2, C-H···π (open circles, the C5H5−···HF complex with the F-H proton donating bond is also included there), C-H···B (black squares) and remaining ones, among them CAHB systems (black circles). The hydrogen···Lewis base distance is understood here in the following way: it is the H···B distance for the 3c–4e (three centre–four electron[2]) A-H···B hydrogen bonds. For the C-H···π interactions this is the distance between the H-atom of Lewis acid unit and the carbon atom or the bond critical point of CC bond of the Lewis base. The latter depends on the kind of the bond path characterizing the intermolecular link. Figure 2 shows that for stronger interactions characterised by shorter distances between Lewis acid-base units the covalent character is revealed that is expressed by the negative HBCP values, the corresponding systems may be treated as the potentially preliminary stages of PT processes. The weaker hydrogen bonds are characterised by longer distances between these units, these are mainly the C-H···π and C-H···B systems.
Figure 2. The dependence between the H···Lewis base distance (between H-atom of the proton donor and the centre of Lewis base unit, in Å) and the total electron energy density at BCP, HBCP for hydrogen bonded systems. The Lewis base centre is an atom for 3c–4e A-H···B hydrogen bonds (black squares for C-H···B hydrogen bonds and black circles for other 3c–4e systems) and it is BCP of CC bond or C-atom in Lewis base unit for the A-H···π hydrogen bonds (while circles).
It is worth to note that other Lewis acid - Lewis base interactions may be treated as preliminary stages of various reactions, for example, the tetrel bond often leads to the SN2 reaction [19] while the dihydrogen bond to the release of the molecular hydrogen.[20]
This entry is adapted from the peer-reviewed paper 10.3390/molecules25204668
References
- Jeffrey, G.A.. An Introduction to Hydrogen Bonding; Oxford University Press: New York, 1997; pp. 117-213.
- Grabowski, S.J.; What is the Covalency of Hydrogen Bonding?. Chem. Rev. 2011, 11, 2597–2625, .
- Pauling, L.. The Nature of the Chemical Bond; Cornell University Press: New York, 1960; pp. 449-504.
- Jeffrey, G.A.; Saenger, W.. Hydrogen Bonding in Biological Structures; Springer: Berlin, 1991; pp. 15.
- Grabowski, S.J.. Understanding Hydrogen Bonds; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1-40.
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S.; Perspectives on halogen bonding and other σ-hole interactions: Lex parsimoniae (Occam’s Razor). Comput. Theor. Chem. 2012, 998, 2-8, .
- Politzer, P.; Murray, J.S.; Clark, T.; Halogen bonding and other σ-hole interactions: A perspective. Phys.Chem.Chem.Phys. 2013, 15, 11178–11189, .
- Bürgi, H.B.; Stereochemistry and Reaction Paths as Determined from Crystal Structure Data – A Relationship between Structure and Energy. Angew. Chem. Internat. Ed. 1975, 14, 460-473, .
- Bürgi, H.B.; Dunitz, J.D.; From Crystal Statics to chemical dynamics. Acc. Chem. Res. 1983, 16, 153-161, .
- Grabowski, S.J.; Krygowski, T.M.; The proton transfer path for C=O∙∙∙H-O systems modelled from crystal structure data. Chem. Phys. Lett. 1999, 305, 247-250, .
- Wong, R.; Allen, F.H.; Willett, P.; The scientific impact of the Cambridge Structural Database: A citation-based study. J. Appl. Cryst. 2010, 43, 811–824, .
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P. Ward, S.C.; The Cambridge structural database. Acta Cryst. 2016, B72, 171-179, .
- Wilson, C.C. . Single Crystal Neutron Diffraction From Molecular Materials; World Scientific Publishing Co. Pre. Ltd.: Singapore, 2000; pp. 22-30.
- Luger, P.. Modern X-Ray Analysis on Single Crystals, 2nd fully revised and extended edition; Walter de Gruyter: Berlin, 2014; pp. 154-239.
- Robertson, J.M.; Ubbelohde, A.R.; Structure and thermal properties associated with some hydrogen bonds in crystals. I. The isotope effect. Proc. R. Soc. London. Ser. A. 1939, 170, 222-240, .
- Benedict, H.; Limbach, H-H.; Wehlan, M.; Fehlhammer, W-P.; Golubev, N.S.; Janoschek, R.; Solid State 15N NMR and Theoretical Studies on Primary and Secondary Geometric H/D Isotope Effects on Low-Barrier NHN-Hydrogen Bonds. J. Am. Chem. Soc. 1998, 120, 2939-2950, .
- Bader, R.F.W.. Atoms in Molecules, A Quantum Theory; Oxford University Press: Oxford, UK, 1990; pp. 1-456.
- Grabowski, S.J.; Lipkowski, P.; Characteristics of XH…π Interactions: Ab Initio and QTAIM Studies. J. Phys. Chem. A 2011, 115, 4765-4773, .
- Grabowski, S.J.; Tetrel bond - σ-hole bond as a preliminary stage of the SN2 reaction. Phys.Chem.Chem.Phys. 2014, 16, 1824–1834, .
- Bakhmutov, V.I. . Dihydrogen bonds, principles, experiments, and applications; John Wiley & Sons: Hoboken, New Jersey, 2008; pp. 192-229.