Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dermatology
Wound healing is highly specialized dynamic multiple phase process for the repair of damaged/injured tissues through an intricate mechanism. Various emerging and innovative strategies for promoting quality wound healing have been discussed.
- wound healing
- stem cells
- 3D bioprinting
1. Nanotherapeutics-Based Strategies
Owing to its complex pathophysiology and associated local and generalized complications, chronic wound management is far more challenging than acute wounds. Earlier approaches utilized the systemic administration of antimicrobial agents, antibiotics, and other local application of drugs in order to improve the wound healing of hard-to-heal wounds. However, these approaches are associated with various shortcomings such as low, or sub-optimal penetration of drugs into deeper skin tissues and the development of bacterial resistance with prolonged usage of antibiotics [95,96]. Therefore, the adoption of newer therapeutic modalities that can eradicate the risks of systemic microbial infection as well as augment drug delivery to the deeper tissues in chronic wounds is a prerequisite for quality wound healing. The emerging therapeutic options include nanotherapeutics, stem cell therapy, phototherapy, and different biological therapies such as microbiome therapy, reactive oxygen species (ROS), and NO generators. Among these emerging therapeutic approaches, nanotherapeutic-based strategies have demonstrated exceptional treatment efficacy for enhanced wound healing using different types of nanomaterials [97,98]. In the nanotherapeutics approach, different forms of nanomaterials (nanoparticles, nanofibers, nanogels, nanoemulsion) that are loaded with antimicrobial agents, antimicrobial peptides, growth factors, interferons, and others through the delivery at wound site have been employed for the treatment of different types of wounds. In addition, nanotechnology-based approaches demonstrated the potential to overcome the various obstacles that are associated with conventional wound healing modalities such as sepsis, sub-optimal penetration to deeper skin tissues, and delayed wound healing. The rapid development of nanotechnology in last two decades has opened new avenues for the delivery of drugs, antimicrobial agents, antibiotics, different biomacromolecules (proteins, peptides), growth factors, DNA/RNA, and different therapeutic moieties for chronic wound healing by playing a vital role in controlling microbial infection, inflammation, hemostasis, and promoting cellular proliferation [99,100]. Moreover, nanoparticulate drug delivery systems using different nanocarriers provide sustained and controlled delivery of a wide range of drugs/therapeutic agents at an optimal concentration with an extended half-life, improved bioavailability, improved pharmacokinetic profiles, and decreased drug administration frequency [101,102]. The therapeutic role of nanocarriers in wounds is attributed to its small size (ranging from 10 to 100 nm) and physicochemical properties, which allows intracellular drug delivery, maintenance of a moist environment with enhanced penetration, and degradation stability [33,103]. Additionally, high encapsulation efficiency of nanocarriers of different drugs and biomolecules increases the effectiveness of delivery for wound healing and skin regeneration applications [104]. Nanotherapeutic-based strategies provide several advantages for controlling the microbial infections in chronic wounds. The advantages include an improved half-life and bioavailability of drugs, improved penetration of drugs into bacterial biofilms and tissue barriers, enhancement of interactions between drugs and microbes, and the ability to increase the concentration of drugs at infection sites [105]. There are two main categories of nanomaterials that are being utilized with distinct properties and efficacy for the management of chronic wounds [98,102]. The first category includes organic nanomaterials which comprises of polymeric nanoparticles, nanogels, nanofibers, nano emulsions, micelles, liposomes, nanosheets, ethosomes, and solid-lipid nanoparticles. The second major category of nanomaterials include inorganic nanomaterials viz., metal nanoparticles, quantum dots, carbon nanotubes, and magnetic nanoparticles [106]. A list of different nanomaterials that are being utilized for wound healing applications are listed in Figure 1.
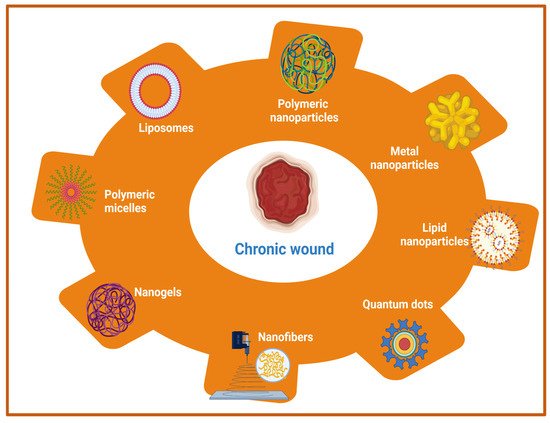
Figure 1. Schematic representation of nanotherapeutic approaches using a wide range of nanomaterials for chronic wound healing.
Furthermore, nanoparticles in wound healing applications are classified into two categories (1) nanoparticles with intrinsic properties for acceleration of wound healing, and (2) nanoparticles as drug delivery systems. Moreover, nanoparticles can be bioengineered in such a way that they can be used either as a therapeutic device or as drug delivery vehicles for the sustained and controlled delivery of various therapeutic molecules for improved wound-healing processes. Owing to the presence of various advantages, metal nanoparticles, metal oxide nanoparticles, polymeric nanomaterials (nanoparticles, nanofibers, nano emulsion), and other nanotherapeutics have been widely employed for the treatment of chronic wounds. Metallic- and metal oxide-based nanoparticles such as silver, gold, zinc, zinc oxide (ZnO), aluminum oxide, titanium dioxide, iron oxide, copper oxide, and gallium possess outstanding antibacterial properties that help in the stimulation of wound healing [107,108,109]. The anti-bacterial activities are attributed to the production of ROS, inhibitory enzymes, damaging effects on bacterial DNA, and biofilms breaking ability to inhibit the bacterial growth [110]. In addition, other materials such as cerium, nitric oxide (NO) nanoparticles, bioactive glass, and carbon-based nanoparticles with intrinsic bioactivity have been utilized for chronic wound management [111]. However, toxicity that was induced by metal-based nanotherapeutics should be considered beforehand otherwise it’s in vivo applications would be restricted. In order to overcome this problem, the optimization of size and properties of metal nanotherapeutics would provide benefits in terms of reducing toxicity. Other than metal-based nanotherapeutics, polymeric nanomaterials with inherent biocompatibility and biodegradability are pushing the forefront for wound healing applications. Polymeric nanotherapeutics include polysaccharide-based polymers, protein-based polymers, polyester-based polymers, polyamide-based polymers, cationic polymers (chitosan), etc. The different types of polymeric nanomaterials include nanoparticles, nanofibers, nanoemulsion, nanosheets, nanogels, polymeric micelles, liposomes, and others for wound healing and skin regeneration [112]. Another crucial strategy using polymeric nanotherapeutics includes the encapsulation of antibiotics/growth factors within polymeric nanomaterials to prevent wound infection and thus shorten the wound healing time [113]. A comprehensive list of nanotherapeutic-based strategies using different organic and inorganic nanomaterials for the treatment of acute and chronic wounds are summarized in Table 1.
Table 1. A list of recent nanotherapeutic approaches for wound healing applications along with wound type being treated, loaded drugs/growth factors, and findings.
| Type of Nanomaterials | Wound Type | Drugs/Therapeutic Agents/Growth Factors | Findings | Reference |
|---|---|---|---|---|
| Poly (ethylene terephthalate) (PET) nanofibers | Acute (skin wound) | Anionic antibiotics piperacillin/tazobactam (PT) | High loading efficiency and sustained delivery for PT, reduced bacterial load | [116] |
| Poly (lactic-co-glycolic acid)/gelatin (PLGA)/gelatin nanofibers | Chronic (diabetic wound) | Liraglutide (Lira) | Shorter wound closure time, enhanced collagen deposition and alignment, increased blood vessel density | [117] |
| Poly (lactic-co-glycolic acid)-polyethylenimine nanoparticles | Acute (skin wound) | Nitric oxide (NO) | Strong bactericidal effect against methicillin-resistant Staphylococcus aureus (MRSA) bacteria, accelerated wound healing | [118] |
| α-gal nanoparticles | Chronic (diabetic wound) | ----------------------- | Enhanced vascularization, re-epithelialization, granulation tissue formation, accelerated wound healing | [119] |
| Solid lipid nanoparticles | Chronic wound | Serpin A1 (A1) and host defense peptide LL37 | Promotion of wound closure, reduction of bacterial contamination, and enhancement of anti-inflammatory activity | [120] |
| Liposome with silk fibroin hydrogels | Chronic (deep second-degree scald) | Basic fibroblast growth factor (bFGF) | Accelerated the wound closure, induced regeneration of vascular vessel | [121] |
| Photoluminescent gold nanodots | Acute (skin wound) | Antimicrobial peptide (surfactin; SFT), and 1-dodecanethiol (DT) | Enhanced antimicrobial properties and collagen deposition | [122] |
| Peptide dendrimers | Chronic (diabetic wound) | ----------------------- | Smaller wound area percentage, improved wound healing | [123] |
| Fusidic acid nanoemulsion | Chronic (burn wound) | ----------------------- | Reduction in bacterial load, wound contraction, and faster re-epithelialization | [124] |
| Recombinant human hair keratin nanoparticles | Acute (dermal wound) | ----------------------- | Improved epithelialization, vascularization, along with collagen deposition and remodeling. | [125] |
| Chitosan nanoparticles | Chronic (prostatic wound) | Rebamipide | Improved re-epithelialization and faster wound healing | [126] |
| PLGA-liposome nanofibers | Acute (skin wound) | MicroRNA 145 (miR-145) and platelet-derived growth factor (PDGF) | Promotion of wound healing with enhanced vascularization and decreased wound size | [127] |
| Gelatin nanofibers | Chronic (burn wound) | anionic drug and hydrotalcite | Accelerated wound healing with strong antimicrobial activity | [128] |
| Silk fibroin nanoparticles | Chronic (ulcerative colitis) | Resveratrol | Reduced level of intracellular ROS, polarization of macrophages to type M2, restoration of damaged colonic epithelial barriers, reduced inflammatory reactions and level of intracellular ROS. | [129] |
| Poly (l-lactic acid) (PLLA) nanofibers | Chronic (diabetic wound) | Silica nanoparticles and dimethyloxalylglycine | Improved neo-vascularization and re-epithelialization with enhanced collagen deposition | [130] |
| Poly-(1,4-phenyleneacetone dimethylene thioketal) | Acute (full-thickness skin defect) | Stromal cell-derived factor-1α(SDF-1α) | Induction of wound vascularization, accelerated wound healing | [131] |
| Elastic liposomes with hyaluronic acid | Chronic (diabetic wound) | Epidermal growth factor (EGF), platelet-derived growth factor-A (PDGF-A), and insulin-like growth factor-I (IGF-I) | Reduction of wound size, improved skin permeation, and healing | [132] |
| Chitosan capped silver nanoparticles | Chronic (burn wound) | ----------------------- | Shortening of the length of repair phases, enhanced re-epithelialization | [133] |
| Polyvinyl alcohol nanogels | Acute (skin wound) | Cerium oxide nanoparticles | Antimicrobial activity and rapid healing | [111] |
| Copper nanoparticles | Chronic wound | ----------------------- | Increased vascularization, accelerated healing process | [134] |
| Chitosan hydrogels | Chronic (diabetic wound) | Silver nanoparticles | Promotion of antibacterial activity, enhanced healing | [135] |
| Polymeric composite dressings | Chronic (diabetic wound) | Calcium | Stimulated angiogenesis, collagen synthesis, accelerated wound healing | [136] |
| Fibrin nanoparticles | Acute (dermal wound) | Keratinocyte growth factor | Better cell proliferation and migration along with enhanced wound healing | [137] |
| Chitosan/Collagen blended nanofibers | Acute (full thickness skin wound) | Curcumin | Reduction in wound coverage area, improved healing | [138] |
| Collagen mats | Chronic wound | Inorganic polyphosphate (polyP) | Reduction in wound area, accelerated re-epithelialization rate and healing | [139] |
Another feasible nanotherapeutics strategy for the treatment of chronic wounds involves the regulation of the inflammatory state using different nanomaterials. In the case of burn wounds, mainly TNF-α, IL-1β, and IL-6 are overexpressed as inflammatory factors, while IL-18 is highly expressed in the case of diabetic wounds. Therefore, the careful regulation of the inflammation phase would be required as inflammatory markers vary in different types of chronic wounds. Silver nanoparticles and polymeric nanofibers demonstrated a reduction in the levels of inflammatory markers IL-1β and IL-6 but not IL-18, suggesting its poor anti-inflammatory effects against diabetic wounds [114,115]. Another limitation includes the difficulty in the identification of the inflammatory phase as the inflammation phase overlaps with the proliferation phases. Therefore, there is a need to develop different nanomaterials with outstanding anti-inflammatory properties for the immunoregulation of different types of chronic wounds and their treatment. In this context, the identification of markers that specifies the transition from the inflammatory phase to the proliferation phase using immunomodulatory nanomaterials would be a feasible strategy. Overall, nanotherapeutic-based strategies present promising approaches for the clinical treatment of chronic wounds by providing excellent antibacterial effects, reduced bacterial drug resistance, reducing the inflammatory phase, and shortening of the wound healing time for the promotion of wound healing.
2. Stem Cell Therapy-Based Strategies
In recent years, regenerative medicine has emerged as a revolutionary field to provide alternative therapeutic strategies to restore normal skin architecture and improve wound healing [140,141]. Stem cell-based therapy regenerative medicine for wound healing and skin regeneration has garnered much interest owing to its properties viz., long-term self-renewal capacity, and differentiation potential to multiple cell types [142]. Among cell therapies for wound healing, stem cells and progenitor cells have received much attention and remaining stem cells that are close to wounds site heal the wounds due to the stem cell’s plasticity. Moreover, stem cell-based therapy has provided great potential for healing chronic wounds which otherwise cannot be healed using conventional therapies. The stem cell-based therapy for chronic wound healing utilizes various processes such as interactions and actions of growth factors, the regulation of inflammatory processes, and the stimulation of immune processes for accelerating the vascularization and re-epithelialization [143]. A number of clinical and preclinical trials using stem cells in recent decades have presented profound impacts on quality wound healing [18,144]. The therapeutic potential of stem cell-based wound therapy is largely attributed to its ability to secrete pro-regenerative cytokines and growth factors for the promotion of skin regeneration during the treatment of chronic wounds [141]. In addition, autologous stem cells have excellent differentiation potential, support angiogenesis, and are well-tolerated by the patient with minimum adverse reactions. The tremendous ability of stem cells to transform into any other types of cells makes them a perfect choice to support the natural healing process to promote cell proliferation and accelerate wound closure. Earlier reports demonstrated the beneficial role of stem cells in wound healing as they directly and indirectly stimulate the residing cells in skin tissue, release active biomolecules, modulate inflammation, and remodel the ECM [145]. Owing to the presence of these beneficial effects, one can anticipate accelerated wound healing with a prevention of wound contraction and scar formation, expeditious wound closure, and regeneration with administration of stem cells. Nevertheless, the main clinical focus of stem cell-based therapy is to target improved quality wound healing for wound care.
Among the different types of stem cells, adult mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and the more recently explored induced pluripotent stem cells (iPSCs) present the main sources of stem cells that are utilized for wound healing and skin regeneration [146]. ECSs as a stem cell source were minimally utilized for wound healing due to the associated ethical concerns. A representative list of the different sources of stem cells that are utilized in wound healing is shown in Table 2. MSCs remain most convincing source of stem cells for wound healing owing to its ability to modulate inflammation, enhanced angiogenesis and granulation tissue formation, antimicrobial effects, reduction of scars, and promotion of fibroblasts [147,148]. MSCs are obtained from a plethora of sources such as bone marrow, adipose tissue, umbilical cord blood, Wharton’s jelly stem cells, and amniotic fluid. MSCs are involved in all the four phases of wound healing for the promotion of wound healing by facilitating migration to the wound site and the stimulation of angiogenesis, growth factors/cytokines release, and re-epithelialization. Bone marrow-derived mesenchymal stem cells were utilized for the first ever human study for the treatment of severe skin burns injuries followed by skin grafting and reported improved neo-vascularization and relief from the pain [149]. In another similar study with burn wounds, patient’s own bone marrow stem cells (BMSCs) were transplanted to the wound surface and treated burn patients involving 80% total body surface area (TBSA) and hypertrophic scarring followed by covering with acellular dermis support matrix [150]. The transplantation of stem cells resulted in reduced wound contraction, modulation of ECM, and enhanced angiogenesis. Furthermore, another clinical study was performed using biodegradable collagen membrane (Coladerm) and autologous bone marrow-derived MSCs and skin fibroblasts for the treatment of chronic non-healing wounds (diabetic ulcer). The results revealed a decrease in the wound size and enhanced vascularization after 29 days of combination treatment [151].
Table 2. A representative list of different stem cells-based therapies for accelerated wound healing.
| Source of Stem Cells | Type of Wounds | Findings | Reference |
|---|---|---|---|
| Bone marrow-derived stem cells | Acute (full thickness wound) | Administration: intradermal and intravenous. Significant improvement in inflammation phase shortening, overexpression of proliferation markers (Ki67, CD71, and CD90), collagen deposition, and granulation tissue re-organization | [152] |
| Bone marrow-derived stem cells and their extracellular vesicles (EVs) | Acute (full thickness wound) | Administration: chitosan/collagen scaffold delivery system. Accelerated wound healing, enhanced collagen deposition | [153] |
| Bone marrow-derived stem cells | Chronic (diabetic wound) | Administration: subcutaneously. Improved collagen deposition and wound healing | [154] |
| Adipose-derived stem cells derived exosomes | Chronic (diabetic wound) | Upregulation and downregulation of specific micro RNAs (miRNAs), Inhibition of inflammation, modulation of PI3K/AKT signaling pathway | [155] |
| Adipose-derived stem cells | Chronic (full thickness burns wound) | Administration: 3D printed scaffold delivery system. Acceleration wound contraction, faster re-epithelialization and healing | [156] |
| Adipose-derived stem cells | Chronic (diabetic wound) | Administration: hydrogel delivery system. Enhanced neo-vascularization and accelerated wound closure | [157] |
| Hair follicles stem cells | Acute (full-thickness excisional wound) | Administration: intradermal injection. Shorter inflammation phase, function vascularization, enhanced re-epithelialization | [158] |
| Hair follicles stem cells | Chronic (venous leg ulcers) | Administration: direct application-hair skin graft. Significant reduction in ulcer area, improved healing | [159] |
| Hair follicles stem cells | Acute (full thickness skin wound) | Administration: direct application-hair skin graft. Overexpression of prostate cancer-upregulated long noncoding RNA 1 (PlncRNA-1), accelerated epidermal regeneration and wound healing | [160] |
| Induced pluripotent stem cells | Acute (full-thickness skin Wounds) |
Administration: direct topical application. Expedited wound closure, enhanced collagen deposition | [161] |
| Induced pluripotent stem cell-derived exosomes | Chromic (diabetic ulcers) | Administration: direct. Enhanced migration and proliferation of fibroblasts, accelerated wound healing | [162] |
| Induced pluripotent stem cell-derived microvesicles | Chronic (burn wound) | Administration: Local transplantation. Accelerated wound closure, promotion of keratinocytes migration, increased re-epithelialization, | [163] |
In a recent study, the transplantation of MSCs reported improved cutaneous wound healing through paracrine signaling of vascular endothelial growth factor that was released by MSCs [164]. Despite the promising role of MSCs in cell-therapy-based approaches for wound care, there remains a few limitations such as long-term safety and poor viability after implantation. In order to improve the survival of transplanted MSCs after implantation, various strategies have been employed [165]. One of the strategies includes scaffold-based therapeutic strategies along with stem cells. Herein, stem cells to be transplanted were first seeded on a three-dimensional scaffold matrix that was derived either from natural or synthetic polymers followed by delivery to the wound site after transplantation. This approach greatly improved the stem cell survival, preservation of cellular functions, and wound healing using stem cells and decellularized silk fibroin scaffold [166,167]. Kamolz et al. utilized Matrigel with Matriderm for the delivery of MSCs to the wound site [168]. Furthermore, the expansion of this approach incorporated growth factors within the scaffolds to improve the vascularization and wound repair [169]. These reports demonstrate the better wound healing effect using stem cells and scaffold-based delivery systems with the addition of growth factors. Furthermore, several compelling clinical studies using stem cells have been conducted with scaffold-based delivery systems for the acceleration of wound healing in chronic wounds [141,170]. However, only a few studies involving natural and synthetic biocompatible scaffolds and stem cells are FDA-approved. Out of 90 clinical trials that were registered for the evaluation of stem cells healing potential, only 23 trials utilized biomaterial scaffolds for stem cell delivery to the wound site and healing chronic wounds as accessed from www.clinicaltrials.gov (US National Institutes of Health (NIH) (accessed on 20 May 2022). Another strategy to improve stem cell survival rate at the wound site includes genetic modification of the stem cells. In a recent study, BM-MSCs overexpressing TGF-β3 improved the wound healing process with reduced scar formation [21]. In another study, epidermal stem cell overexpressing Cd271 reported increased cell differentiation, proliferation, and migration in a burn wound model [171]. Furthermore, the orientation of MSCs play a vital role in improving wound healing and optimal tissue regeneration. Utilizing this strategy, 3D printed polylactic acid (PLA) that was coated with nanotubes of Halloysite (aluminosilicate clay mineral) demonstrated beneficial effects of MSCs orientation on wound healing [172]. In another study, 3D-printed polycaprolactone scaffold that was coated with nanocellulose and seeded with MSCs revealed the promotion of cellular growth and differentiation for the wound healing process [173]. In recent years, MSCs-derived exosomes, which is source of various growth factors and cytokines proved to be a promising newer stem cell-based strategy for accelerating the wound healing process by promoting cell migration and proliferation, angiogenesis, re-epithelialization, and activating several signaling pathways (such as Wnt/β-catenin, AKT, ERK, and STAT3) [169,174]. Adipose-derived MSCs are also very commonly utilized in wound healing applications due to its high accessibility, minimal invasiveness, and lack of ethical limitations [156,175]. Recently, exosomes that were derived from adipose-derived MSCs have shown accelerated wound healing with a significant increase in the wound closure rate by attenuating the inflammation phase [176,177]. Other MSCs source, umbilical cord blood stem cells (UC-MSC) showed promising therapeutic effects in the treatment of diabetic chronic wounds through higher cell proliferation and the deposition of collagen [178]. Although clinical trials with UC-MSC-based therapy are less, these stem cells present advantages over bone marrow MSCs in terms of high numbers of cells isolation, high cell yield, and senescence retardation [179]. In a recent study, a combination of MSCs with natural substances have demonstrated enhanced healing properties using a combination of human umbilical cord-derived MSCs and platelet-rich plasma cryogels in third degree burn wounds with significantly increased angiogenesis, wound closure, and re-epithelialization compared to controls [180]. Platelet-rich plasma is derived from blood and is rich in growth factors and antimicrobial properties that are a prerequisite for the improvement of wound healing. Another stem cells source, human Wharton’s jelly stem cells (HWJSC), also reported better healing in burn wounds when transplanted with acellular dermal matrix [181].
Apart from these MSCs sources, there are various other stem cells that are harbored by skin that accelerate wound healing. The epidermis harbors different stem cells such as bulge area stem cells, interfollicular stem cells, and sebaceous gland stem cells [182]. On the other hand, the dermis contains two types of stem cell populations viz., hair follicle stem cells in dermal papilla and perivascular stem cells. These stem cells are reported to accelerate the wound healing process with minimum ethical concerns and immune rejections [158]. However, in order to have more substantial understanding regarding their usefulness in wound healing, the exploration of more clinical trials is required in humans to confirm the effects.
In order to overcome the shortcomings of MSCs and ESCs, a new stem cells source, induced pluripotent stem cells (iPSCs) has been unleashed in the regenerative medicine domain [125,141,183]. iPSCs are remarkably similar in morphology with ESCs but without any ethical concerns and potential for immunological rejection that is associated with ESCs [184]. iPSCs are reprogrammed cells that are derived from somatic cells combination of reprogramming factors (e.g., Sox2, Klf4, Oct3/4, and c-Myc) and are considered as a rising star in regenerative medicine and opened unprecedented opportunities for transplant therapy. Bilousova et al. demonstrated differentiation of iPSCs into epidermis, sebaceous glands, and hair follicles [185]. In another study, epithelial stem cells that were derived from iPSC (iPSC–EpSC) indicated reconstitution of hair follicles [186]. Furthermore, various research studies have demonstrated either iPSCs or iPSCs-derived exosomes for the facilitation of wound healing with enhanced wound closure, collagen synthesis, and angiogenesis [162,187,188]. In recent studies, iPSCs from different somatic cells such as fibroblasts, keratinocytes, and folliculogenic human epithelial stem cells showed significant progress in bioengineering of skin substitutes [146]. Furthermore, Yan et al. utilized iPSCs- derived micro-vesicles containing microRNA (miR-16-5p) for enhanced re-epithelialization and collagen deposition in burn wounds [163]. The iPSCs-based stem cells strategy can be combined with genome editing tools for permanent corrective therapy for chronic injuries using clustered, regularly interspaced, short palindromic repeats (CRISPR), transcription activator-like nuclease effector (TALEN), and zinc nuclease finger (ZFN) tools. Overall, stem cell-based therapy and different strategies provided an upper hand over conventional approaches for enhanced wound healing. However, the exploration of stem cells sources, processing methods, and administration routes should be more studied in order to realize the real clinical situation in regenerative wound healing.
3. D Bioprinting-Based Strategies
In last two decades, several therapeutic modalities have been extensively utilized for the treatment of acute and chronic wounds. However, most of the treatment options were manual and their success was hindered by the requirement of a relatively long time to cover large wounds or burns. To overcome this limitation, 3D bioprinting has emerged as a rapid and high throughput automation technology in recent decades to address these challenges in regenerative medicine, including wound healing. The three-dimensional (3D) bioprinting technique is an additive manufacturing technique that provides a promising strategy for the fabrication of biocompatible artificial skins through a precise layer by layer deposition of living cells, biomaterials, biomolecules, and growth factors. This automated technology is a flexible tool which is superior for clinical uses in terms of accuracy and functionality. The advantages of 3D bioprinted skin constructs for wound healing and skin regeneration include (1) automation and faster fabrication which lesser time and cost; (2) flexibility to introduce different cells and biomolecules during the process for the promotion of innervation, pigmentation, and vascularization; (3) the ability to precisely deposit multiple biomaterials and cells in different positions; and (4) large-scale fabrication with good plasticity and extensibility [189,190,191]. The bioprinted skin equivalents closely mimic the native skin architecture and heterogenicity via the precise deposition of multiple cells and biomaterials [192]. The bioprinted skin constructs should fulfill various features in terms of functional and compositional properties. Firstly, bioprinted skin substitutes should be able to transport nutrients and wound exudates. Secondly, bioprinted equivalents should be able to accurately deposit different skin cells such as keratinocytes, fibroblasts, adipocytes, melanocytes, Langerhans cells, etc., at particular layers and locations. Thirdly, porosity, degradation, and mechanical properties should closely mimic the native skin structure, and finally, the bioprinted structure should be robust, biocompatible, biodegradable, and should be able to sustain external forces and pressures that are present in in vivo conditions [193,194]. Furthermore, with the recent advancement in 3D bioprinting technologies it may be utilized for the development of an in vitro 3D disease model, and high throughput platform for drug screening (Figure 2). Moreover, a multi-thronged approach using application-specific bioprinting systems is required to develop personalized therapies for different types of wounds using autologous cells.
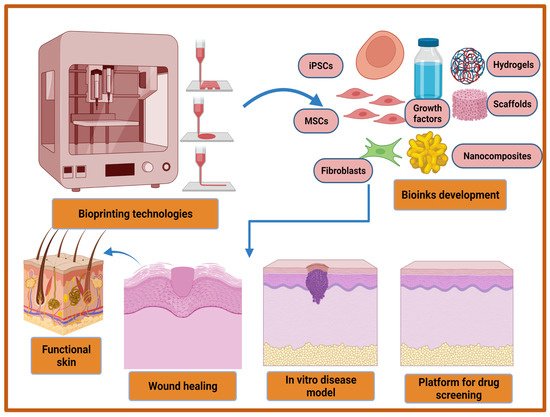
Figure 2. Recent advancements in 3D bioprinting technologies and bio-inks development for improved wound healing, in vitro disease model development, and the fabrication of high throughput platform for drug screening.
There are mainly four different types of bioprinting technologies that are being utilized for cutaneous wound healing and skin tissue regeneration. The different types of bioprinting techniques based on their native prototypes include extrusion-based, droplet-on-demand (inkjet)-based, laser-based, and stereolithography-based bioprinting technologies [195,196,197,198]. Among all these technologies, extrusion-based bioprinting (EBB) techniques are the most common for skin bioprinting owing to different advantageous features such as high printing speed, cost-effectiveness, accessibility, capability to replicate complex tissues, and the ability to print wide range of viscous biomaterials [199]. In EBB, to deposit the predesigned geometrically well-defined 3D complex structures of skin, the extrusion of continuous stands of bio-inks through a nozzle is generally powered by pneumatic pressure, screw-based, piston, or microfluidic mechanisms [200]. However, the EBB technique suffers from one limitation that is frequent clogging problems with different types of nozzles. Similarly, other abovementioned bioprinting techniques also have various pros and cons that need improvement in terms of bioprinting device designs and methodologies. In order to overcome the limitations of bioprinting technologies, newer and/or improved bioprinting technologies and the utilization of hybrid bioprinting systems combining two different existing bioprinting systems would be prospective strategies for skin bioprinting. The examples of new and improved forms of existing bioprinting technologies includes continuous liquid interface production (CLIP) [201], multi-material multi-nozzle 3D printing (MM3D) [202], and computed axial lithography (CAL) [203]. CLIP-based bioprinting is better than conventional stereolithography (SLA) printing methods as it has 100 times faster printing speed and creates a ‘no polymerization zone’ using an oxygen-permeable window below the UV projection to ensure continuous SLA process [201]. CLIP can be extended for printing multi-layer skin constructs on a larger scale using different photo-initiators, visible light-based techniques, and biocompatible photopolymers. MM3D-based bioprinting employs a voxel-by-voxel approach instead of the conventional layer-by-layer approach using a specialized printhead with high frequency and ability to switch between up to eight different materials [202]. This bioprinting approach provides benefits to skin tissue printing as it allows the maintenance of stiffness difference, not only between the different layers but also within length and breadth of any single layer. This method of bioprinting and CAL was developed in 2019 much later than CLIP in 2015 for skin bioprinting applications. Therefore, much exploration is still required using this bioprinting technology for skin tissue regeneration. The other revolutionary bioprinting method includes CAL, which utilizes volumetric construction instead of conventional layer-by-layer construction for 3D bioprinting [203]. The advantages of CAL include the utilization of visible light instead of UV light or laser light, and printing flexibility over a preexisting solid object. Besides the new or improved bioprinting techniques, another approach to improve skin bioprinting includes the utilization of hybrid bioprinting systems, which involves two or more existing bioprinting techniques. For example, Kim et al. have demonstrated the construction of epidermal and dermal skin layers by using inkjet and extrusion bioprinting techniques, respectively [204]. Moreover, hybrid bioprinting-based approaches hold great potential for skin bioprinting and needs further exploration in order to realize its full potential.
The other important criteria for successful wound healing and skin engineering using 3D bioprinting techniques include biomaterial ink or bio-ink [205,206]. Bio-inks are a combination of biomaterials, cells, and growth factors/biomolecules, while biomaterial ink comprises of everything that is present in bio-ink but cells. A wide range of biomaterial hydrogels are being employed for skin bioprinting viz., chitosan, gelatin, collagen, silk fibroin, alginate, cellulose, hyaluronic acid etc., along with synthetic biopolymers to improve the mechanical properties of bioprinted constructs [207,208]. Whether natural or synthetic biopolymers are used as bio-inks, there are certain crucial properties that should be possessed by all bio-inks. These properties include biocompatibility, high availability, biodegradability, good printing ability, mechanical stability, and the ability to maintain high shape fidelity after the bioprinting process. In addition, bio-inks utilize living cells which can impact the immune response after implantation, therefore, the selection of cells should be considered keeping in mind this crucial point. In skin bioprinting, either primary skin cells such as fibroblasts, melanocytes, keratinocytes, or stem cells are preferred for the fabrication of skin constructs while maintaining all cellular activities after bioprinting [209]. Another important property of bio-ink includes viscoelastic behavior that influences the skin bioprintability and also affects the cell migration, proliferation, and remodeling of the ECM [210]. Regardless of the bio-inks or bioprinting techniques that are utilized, skin bioprinting for wound healing applications is carried out using two strategies, namely ex vivo and in situ bioprinting. In ex vivo bioprinting, an extrusion-, inkjet-, or laser- based bioprinting method is utilized for printing epidermal and dermal layers of the skin followed by in vitro maturation, if necessary. After maturation, the bioprinted skin constructs are grafted to the wounds of the patient. On the other hand, bio-inks (containing skin cells in biomaterial hydrogel) are directly bioprinted on the injured site in an in situ bioprinting approach using the most common handheld extrusion bioprinter to reproduce the skin structure followed by bio-inks crosslinking, if required [211].
Lee et al. reported the first bioprinted skin in 2009 using collagen hydrogel and human dermal fibroblast-based bio-ink [212]. Furthermore, Koch et al. fabricated skin equivalents by utilizing a collagen hydrogel-based bio-ink containing keratinocytes and fibroblasts [213]. At the same time, Binder et al. employed a 3D inkjet-bioprinter using human fibroblasts and keratinocytes for fabricating skin substitutes for wound healing [214]. Afterwards, significant progress was reported in the skin bioprinting filed using a wide range of bio-inks and bioprinting techniques. 3D bioprinted skin equivalents serve as an alternative to traditional skin grafts for the regeneration of skin tissue structure with appendages and overcome the problem of donor and surgery requirements [189,215]. Moreover, this emerging skin bioprinting technology has been applied for the treatment of various chronic and non-healing wounds such as burn wounds, pressure ulcers, venous ulcers, and diabetic foot ulcers. In a study, a hand-held 3D bioprinting instrument was developed for the treatment of full thickness burn wounds in porcine models with reduced scar formation [216]. This hand-held bioprinter allows in situ bioprinting of sheets of skin tissue, maintaining the cells heterogenicity and compositional variations in different skin layers. This system utilizes epidermal and dermal skin cells and different biopolymers such as collagen, alginate, and hyaluronic acid hydrogels for skin bioprinting. In another study, full-thickness human skin equivalent with structural and biomechanical similarity to native skin were fabricated along with an undulated pattern of the unique dermal-epidermal junction [217]. In the last decade, tremendous progress has been witnessed in the skin bioprinting field [217,218,219]. Using 3D bioprinting technologies, initially only dermis printing [220] was attained followed by bilayered dermal and epidermal layer bioprinting [217,221,222], and skin trilayers bioprinting (epidermis, dermis, and hypodermis) [223,224]. Table 3 summarizes some of the most important studies using 3D bioprinting technology and constructs for accelerating wound healing and skin regeneration. The tremendous progress in skin bioprinting is attributed to exploration of newer and improved bio-ink formulations, availability of a wide range of bioprinters with different capabilities and properties, tremendous progress in bioprinting technologies, and the escalation of cell biology and metabolism knowledge before, during, and after the bioprinting process.
Table 3. A representative list of bio-inks and bioprinting methods for wound healing applications.
| Biomaterial/Bioink/Cells | Bioprinting Method | Type of Wound | Findings | Reference |
|---|---|---|---|---|
| Fibrin and collagen hydrogel (Fibroblasts and keratinocytes) |
In situ extrusion bioprinting | Acute (full thickness skin wound) | Rapid wound closure, reduced contraction, and accelerated re-epithelialization. |
[215] |
| Fibrin hydrogel with gelatin, glycerol, and hyaluronic acid (Keratinocytes, melanocytes, fibroblasts, follicle dermal papilla cells, and microvascular endothelial cells, preadipocytes.) |
Extrusion bioprinting | Acute (full thickness skin wound) | Accelerated wound closure, promotion of epidermal barrier formation, reduction in wounds contraction, remodeling of collagen |
[223] |
| Gelatin/sodium alginate/gelatin methacrylate hydrogel (Dermal fibroblasts and epidermal keratinocytes) |
Extrusion bioprinting | Acute (full thickness skin wound) | Reduced wound contraction and scarring, enhanced skin epithelialization, accelerated wound healing | [225] |
| Plasma-derived fibrinogen-containing factor XIII, fibronectin, thrombin, and macrophages (FPM bioink) (Primary fibroblasts human endothelial cells, and keratinocytes) |
Extrusion bioprinting | Acute (full thickness skin wound) | Rapid wound closure and facilitation of re-epithelialization process | [226] |
| Fibrinogen/collagen hydrogel (Fibroblasts and keratinocytes) |
In situ inkjet bioprinting | Acute (full thickness skin wound) | Improved wound closure and re-epithelialization process | [214] |
| Fibrin-collagen hydrogel (Amniotic fluid-derived stem (AFS) cells and bone marrow-derived mesenchymal stem cells (MSCs) |
In situ extrusion bioprinting | Acute (full thickness skin wound) | Enhanced angiogenesis and wound closure rates | [227] |
| Skin-derived extracellular matrix (S-dECM) bio-ink (Fibroblasts, keratinocytes, endothelial progenitor cells and adipose-derived stem cells (ASCs) |
Extrusion and inkjet bioprinting | Acute (full thickness skin wound) | Accelerated wound closure, enhanced re-epithelization, and neovascularization | [221] |
| Living photosynthetic microalgae scaffolds | In situ bioprinting | Chronic (diabetic wound) | Significantly reduced local hypoxia, accelerated chronic wound closure increased angiogenesis, and enhanced extracellular matrix (ECM) synthesis | [228] |
| Sodium alginate/gelatin/collagen hydrogel (Fibroblasts and keratinocytes) |
Extrusion bioprinting | Acute (full thickness skin wound) | Enhanced re-epithelialization, reduced skin wound contraction, and accelerated wound healing | [229] |
| Strontium silicate (SS) microcylinders (Fibroblasts and keratinocytes) |
Extrusion bioprinting | Acute and chronic wounds | Outstanding angiogenesis and wound healing | [230] |
4. Extracellular Matrix (ECM)-Based Strategies
During wound healing, the matrix of the dermal layer generally directs all the phases of healing process after skin damage. However, in the case of chronic wounds, healing is stalled due to a lack of functional ECM in the dermal matrix, which is responsible for stimulating healing with the aid of external factors [234]. The presence of highly activated matrix metalloproteinases, increased abundance of senescent fibroblasts, and a persistent inflammatory phase in chronic wounds degrades the ECM which causes poor wound healing [235]. The restoration of functional ECM in chronic wounds stimulates, directs, and systematizes the healing process for facilitating wound closure. Therefore, in order to improve the healing process in chronic wounds, a proper understanding of ECM components and its interactions with cells is a prerequisite. The ECM is an extensive three-dimensional molecular network, which provides structural integrity and mechanical support to different tissues, including skin. The ECM structure comprises of a variety of macromolecules such as fibrous proteins (collagen, fibronectin, vitronectin, elastin, and laminin), glycosaminoglycans, and proteoglycans [236]. The ECM plays a crucial role in directing the wound healing process by managing cell behavior (adhesion, migration, proliferation, and differentiation) and cellular activities in the damaged skin for tissue repair and regeneration [237,238,239]. In addition, the dynamic ECM structure acts as a reservoir for the storage and delivery of different growth factors and cytokines to provide biological signals. The ECM composition undergoes changes during the wound healing phases and facilitates active interactions of cells and growth factors bioavailability to accelerate the wound healing process [240]. In the hemostasis phase, provisional ECM that is made up of fibrin and fibronectin is formed to support cell adhesion and migration. Furthermore, the ECM provides a 3D structural framework during the inflammation phase for the clearance of microorganisms. Thereafter, provisional ECM is formed during the hemostasis phase disrupts and is replaced with the formation of granulation tissue (fibronectin) to support collagen deposition. Finally, collagen I is replaced with the synthesis of collagen III during remodeling to provide mechanical support. In skin tissue, ECM components communicate with precursor cells and stem cells that are present in skin tissue through integrin and non-integrin receptors during the repair process [240]. The interactions of ECM and stem cell integrins play crucial roles in the wound healing process by modulating various cellular key events. The key events of wound healing such as migration, proliferation, differentiation, and cellular death are mediated through outside-in signaling of integrins. As a result, if the ECM components and stiffness dysregulates, the reciprocal interactions between the cells and ECM and tissue regeneration are ultimately disrupted leading to pathological conditions. Therefore, a better understanding of the ECM components and their roles in the wound healing process may improve the strategies that are related to wound care.
Although the ECM-based approach for the stimulation of wound healing is not very new, how the different ECM components provide a ‘jump-start’ to the healing process are still not much explored. In recent decades, various ECM-based strategies have been employed for the treatment of chronic wounds. The most prominent strategy includes the utilization of bioengineered tissue-derived or synthetic ECM scaffolds to promote wound healing. The ECM-based scaffolds mediate the recruitment of cells, delivers growth factors to injured wound site, and provides mechanical support. There are different types of ECM-based scaffolds viz., cellular-derived ECM [237], synthetic biomaterials-based scaffolds/hydrogels [241,242], and decellularized ECM (dECM) scaffolds [243,244]. Among all the ECM-based approaches, the dECM-based approach presents the most promising approach in recent years as alternative emerging strategies to overcome the hurdles that are associated with the organ/tissue transplantation viz., donor shortage and disease transmission risk. dECM that are either derived from tissues or cells are devoid of cellular components and serves as reservoirs for cell-matrix interactions and site-specific bioactive components, offering various advantages for the regeneration of tissues. The preserved non-immunogenic ECM mimics native three-dimensional architecture and non-immune environment for the repair and regeneration of skin tissues [234]. The skin repair is mediated through the utilization of dECM scaffolds as they retain the porous bilayer structural architecture of skin along with bioactive molecules, elasticity, and adhesiveness of the skin and vessel structure [245,246]. In addition, dECM degradation products do not illicit any toxicity while skin is regenerated and, in fact, promoted the formation of tube-like structures for skin regeneration [247]. In a study, dECM-based scaffolds facilitated the adhesion of keratinocytes due to maintained basement membrane. Additionally, dECM with preserved dermal structure (papillary and reticular dermis) is reported to promote the growth of vascular cells [248,249]. In another study, Choi et al., demonstrated a reduction of wound contraction and less scar formation due to the release of basic fibroblast growth factors from dECM [250]. Furthermore, Brouki Milan et al. demonstrated enhanced cutaneous wound healing as revealed from reduced collagen deposition, faster re-epithelialization, and accelerated wound closure rate using a decellularized dermal matrix (DDM) scaffold reseeded with human umbilical cord perivascular cells (HUCPVCs) [251]. In another study, Groeber et al. reported the fabrication of a bilayared skin alternative that was suitable for skin grafting using decellularized porcine jejunum that was reseeded with dermal fibroblasts, human epidermal keratinocytes, and human dermal microvascular endothelial cells [252]. Owing to dECMs ability to preserve endogenous angiogenesis factor and vasculature, dECM scaffolds have been employed in diabetic pressure ulcers (DPU) treatment wherein high vascularization is required to promote healing [253]. In another study, accelerated wound healing in DPU’s has been reported by shortening the inflammation period, regulating granulation tissue formation, pro-angiogenesis activity, and epithelial regeneration using dECM scaffolds [251]. These regulatory activities that are mediated by dECM scaffolds may reduce the scar formation. In recent years, many acellular products such as AlloDerm® regenerative tissue matrix and Oasis® wound matrix has been commercialized for clinical applications and have shown promising results [254]. However, in order to become skin alternatives, ECM-based acellular products need thorough optimization of fabrication and characterization procedures. In order to attempt this, decellularized human placenta-derived ECM-based scaffolds successfully modulated healing in full thickness wounds along with hair follicle formation [250,255]. The reconstitution of hair follicles and sweat glands is the main criteria for native skin regeneration, which was not observed in earlier clinical products. Overall, decellularized ECM scaffolds and different types of ECM mimics presents great potential for chronic wound treatment and may augment wound healing many folds by incorporating growth factors and antibiotics.
5. Platelet-Rich Plasma (PRP)-Based Strategies
Platelet-rich plasma (PRP)-based endogenous therapeutic technology has the potential to stimulate and accelerate tissue healing including wound healing and has garnered much attention in recent years in regenerative medicine [256,257]. PRP is an autologous biological product containing higher amounts of platelets compared to circulating blood and thus represents an increased concentration of growth factors which is a prerequisite for wound healing [258]. PRP is also called an autologous platelet concentrate, autologous platelet gel, or plasma-rich growth factors and plays a pivotal role in wound healing as platelets have hemostatic function and promote skin cell proliferation and tissue expansion [259]. The growth factors pool in PRP includes platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor beta 1 (TGF-β1), insulin growth factor-1 (IGF-1), keratinocyte growth factor (KGF), vascular epithelial growth factor (VEGF), etc., that promote cell migration, proliferation, and differentiation, for the initiation of wound healing. Growth factors plays pivotal roles in complex wound healing processes and skin tissue regeneration as they act as signaling molecules which influence the cellular metabolism [260]. Almost all cell types that are present in the skin are involved in producing growth factors and different cells produce different types of growth factors during the wound healing phases. Each growth factor demonstrates more than one effect and controls cellular processes such as cell migration and proliferation, ECM remodeling, and angiogenesis which provides an ideal environment and promotes the wound healing process. The paramount importance of growth factors in wound healing lies in the fact that a single growth factor is not sufficient for the treatment of hard-to-heal chronic wounds, especially diabetic foot ulcers [261]. The advantages of using PRP technology in wound healing include easy methodology, safety, and cost effectiveness [262]. PRP is obtained from patients’ blood followed by a simple centrifugation process for its preparation. Therefore, by controlling the centrifugation parameters and the protocol that is being used for the activation it is possible to control the dose of growth factors that are provided by PRP. PRP technology has more long-lasting effects than conventional therapies and it is safer to use as it is obtained from the same patient (autologous). Additionally, it has potential antibacterial activity besides the potential impact in the reduction of economic costs for standard treatment regimens. Moreover, PRP treatment-based therapy does not replace the conventional therapies but serves as an alternative treatment.
In recent years, the usage of PRP technology in wound healing is steadily increasing in various preclinical and clinical studies. The beneficial role of PRP-based treatment in wound healing has been extensively reported in humans [263,264], horses [265], dogs [266], and other species [267,268]. The first clinical application of PRP was performed on chronic leg ulcers using collagen-embedded platelet proteins with the induction of vascularized connective tissue in wounds [269]. Thereafter, different forms of PRP such as gel, solution, or injection have been employed in different wounds with varying etiologies. The different wounds wherein PRP treatment has been reported in various animal and human trials include acute cutaneous wounds [270], chronic skin ulcers [271], burns [272], and plastic and cosmetic surgery [273]. Furthermore, PRP treatment did not elicit any adverse reactions in terms of risk of infection or hypersensitivity reactions during clinical trials [274]. Among the human clinical trials, the main use of PRP treatment is related to hard-to-heal chronic wounds wherein there is an impairment of the wound healing due to a low concentration of growth factors, persistent inflammation, imbalance of pro-inflammatory and anti-inflammatory cytokines, and excess reactive oxygen species [275]. PRP, being rich in different growth factors, have a strong regenerative capacity which shortens the wounds recovery time [262]. In a study, PRP-based dressing treatment accelerated wound healing in skin ulcers and promoted the proliferation and migration of fibroblasts and mesenchymal stem cells (MSC) with faster neovascularization in clinical patients [276]. In another clinical study with 150 patients, the topical application of PRP in diabetic foot ulcers resulted in early complete closure of wounds with the formation of healthy granulation tissue [277]. In other study, the combination of topical application of PRP gel and subcutaneous autologous PRP injections in non-healing wounds demonstrated a significant reduction in the wound size, reduction in pain and inflammation, and potential safety in all the treated patients without any side-effects [263]. Moreover, PRP treatment has successfully demonstrated faster wound healing in different acute and chronic wounds with different etiologies. However, to develop final PRP-based therapeutic product, more standardization and characterization of different PRP preparation methods would be required [278]. In addition, PRP adjunctive treatment has been combined with either cell therapy or skin grafts in various clinical trials and does not delineate the actual potential of only PRP treatment. Nonetheless, recent clinical studies demonstrated improved wound healing outcomes suggesting its therapeutic potential in healing different types of chronic wounds.
6. Cold Atmospheric Plasma Therapy-Based Strategies
Plasma-based treatment strategies have been employed in biomedical engineering for the last 20 years in ulceration and cancer therapy. Plasma represents a fourth state of matter and is classified into thermal and non-thermal (cold) plasma. In thermal plasma, all the particles (electrons and heavy particles) are in thermal equilibrium, while non-thermal or cold plasma contain particles which are not in thermal equilibrium. In recent years, plasma-based therapy for wound healing has gained much attention owing to its beneficial properties. Cold plasmas, also termed as non-thermal plasma, gas plasma, or physical plasma, are most common in biomedical research as it utilizes a lower temperature (<40 °C) which is compatible for biomedical applications. Cold plasma is an ionized gas near room temperature that is comprised of charged particles (electrons, diverse reactive oxygen, and nitrogen species) and neutral particles (neutral atoms and molecules) [279]. In addition, the plasma cocktail contains UV irradiation and an electric filed that in combination with different ions and reactive species mediates biological effects that are necessary for tissue regeneration. The generation of plasma occurs due to a bombardment of electrons and photons with sufficient energy with the neutral atoms and molecules that are present in the gas phase. There are two primary sources for discharge of low-temperature plasma viz., dielectric barrier discharge (DBD) and non-DBD type atmospheric pressure plasma jet (APPJ) [280]. Moreover, cold atmospheric plasma (CAP) acts as an innovative intervention as it employs multimodal mechanisms of action for the treatment of chronic wounds and promotion of wound healing [281]. Cold plasma has the potential to reduce the bacterial load and induce the hemostatic process of wound healing due to the generation of the anti-infective reactive oxygen and nitrogen species. Additionally, cold plasma treatment renders the inactivation of microbial species through the generation of reactive oxygen species (ROS) and nitrogen species (RNS) and demonstrated potential in tissue regeneration in case of wounds. Among reactive nitrogen species, nitric oxide (NO) is most common for bacterial load reduction, promotion of ECM remodeling, and angiogenesis [282]. ROS and NO together mediate the enhanced expression of growth factors in wound regions and ultimately regulate the wound contraction and re-epithelialization [283]. The effects of cold atmospheric plasma on the improvement of wound healing are depicted in Figure 3.
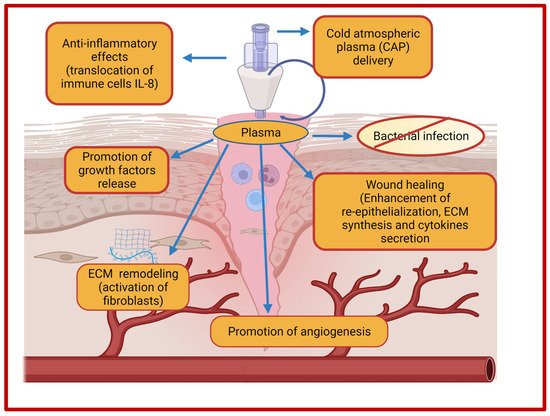
Figure 3. Effects of cold atmospheric plasma (CAP) on wound healing.
The cold plasma utilizes both physical and biological mechanisms for wound healing. The physical mechanism of plasma-based treatment involves the generation of free radicals and reactive species for the desired wound healing-promoting effects, while the biological mechanism exploits various cellular processes that are responsible for DNA and cell membrane damage of bacteria. The advantages of using cold plasma in wound healing includes simplicity in use, non-invasive nature, wound acidification, antimicrobial effects, stimulation of cellular proliferations, enhanced microcirculation, and ultimately reduced treatment costs [279,284,285,286]. Wound acidification promotes the wound healing process due to decreases of pH in the aqueous medium that is induced by cold helium plasma treatment [285]. Currently, there are three certified and approved cold plasma devices for biomedical uses. The first plasma device was certified as a medical device Class IIa in 2013 under the name of kINPen®MED by INP Greifswald/neoplas tools GmbH, Greifswald, Germany) after evaluation in preclinical, clinical, and biological studies. kINPen®MED is a pen-sized atmospheric plasma jet (APPJ) device that was initially developed for laboratory usage but later scaled up also for commercial purposes. The intended use of kINPen®MED included the treatment of acute wounds, infected wounds, pathogen-induced skin diseases, and chronic wounds [287]. Moreover, kINPen MED device represents the largest and best elaborated prospective for diabetic foot ulcers (chronic wounds). Thereafter, a DBD source-based plasma device PlasmaDerm® VU-2010 was developed by CINOGY Technologies GmbH, Germany and CE-certified by MEDCERT under ISO 13485. Consequently, the medical device SteriPlas was developed by Adtec Ltd., London, UK for the reduction of bacterial load and the treatment of acute and chronic wounds [288].
The effect of cold atmospheric plasma treatment on wound healing has been assessed in several in several clinical studies and case reports. The outcomes include the reduction of bacterial load, rapid healing, and faster migration of cells indicating the benefits of the cold plasma treatment in wound care. The ability to generate reactive species such as ROS and RNS for the induction of tissue regeneration provides additional benefits. Several preclinical and clinical studies in humans have been conducted for the treatment of chronic wounds without inducing any substantial side effects on normal tissue [286,289,290,291,292]. CAP treatment facilitated the transformation of chronic wounds to not become stagnating wounds through the modulation of inflammation, tissue-reactive species interactions, and the stimulation of different growth factors [285,293,294]. Fathollah et al. demonstrated accelerated wound healing with increased release of transforming growth factor (TGF)-β1 in diabetic wounds [290]. The researchers found stimulated cell proliferation, neovascularization, and the formation of the epidermis layer after cold plasma treatment. A representative list of the effects of CAP treatment on acute and chronic wounds healing is presented in Table 4 along with the device that was utilized, exposure time, and outcomes. Nevertheless, cold plasma treatment for the prevention of infectious skin diseases and enhanced wound healing holds immense scope for the future. In this context, plasma therapy expanded our knowledge in the domain of enhanced treatment options for wound healing.
Table 4. Representative list of the cold atmospheric plasma treatment-based applications on chronic and acute wound healing.
| Type of Wounds | Number of Patients | Type of Plasma Treatment/Device/Injected Gas/Exposure Time | Findings | Reference |
|---|---|---|---|---|
| Chronic | n = 36 | MicroPlaSter cold plasma alpha device with argon (5 min daily treatment) |
Significant bacterial load reduction (34%) | [295] |
| Chronic | n = 24 | MicroPlaSter cold plasma alpha device, MicroPlaSter cold plasma beta device with argon (2 min daily treatment) |
Significant reduction in bacterial load (40%) | [296] |
| Chronic | n = 70 | MicroPlaSter cold plasma alpha device (3–7 min treatment) | Accelerated wound healing | [289] |
| Acute (wounds present at donor skin graft site) | n = 40 | Cold atmospheric argon plasma, Plasma jet with argon (2 min every day for 1 week) |
Improved re-epithelialization at donor sites | [297] |
| Acute (trauma) | n = 2 | Plasma jet device with electrodes (20 min treatment) | Stopping of wound exudation, improved wound healing | [298] |
| Chronic (venous leg ulcers) |
n = 14 | PlasmaDerm® VU-2010 device (45 s/cm2 for maximum 11 min thrice in a week for 8 weeks) | Strong antibacterial effects, significant reduction in chronic ulcer size | [299] |
| Chronic (venous leg ulcers) | n = 16 | Antiseptic effects of cold atmospheric pressure plasma (APP) or octenidine (OCT) with argon | Significant microbial reduction (64%) without cytotoxicity | [300] |
| Chronic | n = 34 | Tissue-tolerable plasma (TTP) and modern conventional liquid antiseptics | Provided most efficient strategy using antiseptic treatment, highest antimicrobial efficacy, | [301] |
| Chronic (pressure ulcers) | n = 50 | Low-temperature atmospheric-pressure plasma (LTAPP) jet with argon (1 minute/cm2 once in a week for 8 weeks) |
Reduction in bacterial load, significantly better PUSH (Pressure Ulcer Scale for Healing) score | [292] |
| Chronic (diabetic foot) | n = 65 | Argon Plasma Jet (8 times treatment within 14 days) | Reduction in wound surface area, change in microbial load | [302] |
| Chronic (diabetic wounds) | n = 14 | Cold atmospheric plasma | Promotion of vascularization, granulation tissue formation, and re-epithelialization | [286] |
This entry is adapted from the peer-reviewed paper 10.3390/cells11152439
This entry is offline, you can click here to edit this entry!
