Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Phytosterols are functional ingredients with known efficacy and safety. Phytosterols are found as free sterols or as their esters with fatty acids. Although phytosterol esters are soluble in oil and have been used in many commercial foods, it has been difficult to similarly use free phytosterols since they are insoluble in water and practically insoluble in oil. Phytosterols are attractive, functional ingredients for preventing arteriosclerotic diseases since they reduce serum total cholesterol and LDL-cholesterol levels.
- phytosterol esters
- free phytosterols
- Safety
1. Phytosterols
Phytosterols are structurally similar to cholesterol and constitute plant cell membranes [1]. Various phytosterols are found in nature, especially in seeds, major examples of which include β-sitosterol, campesterol, stigmasterol, and brassicasterol [2]. Structures of these phytosterols are shown in Figure 1.
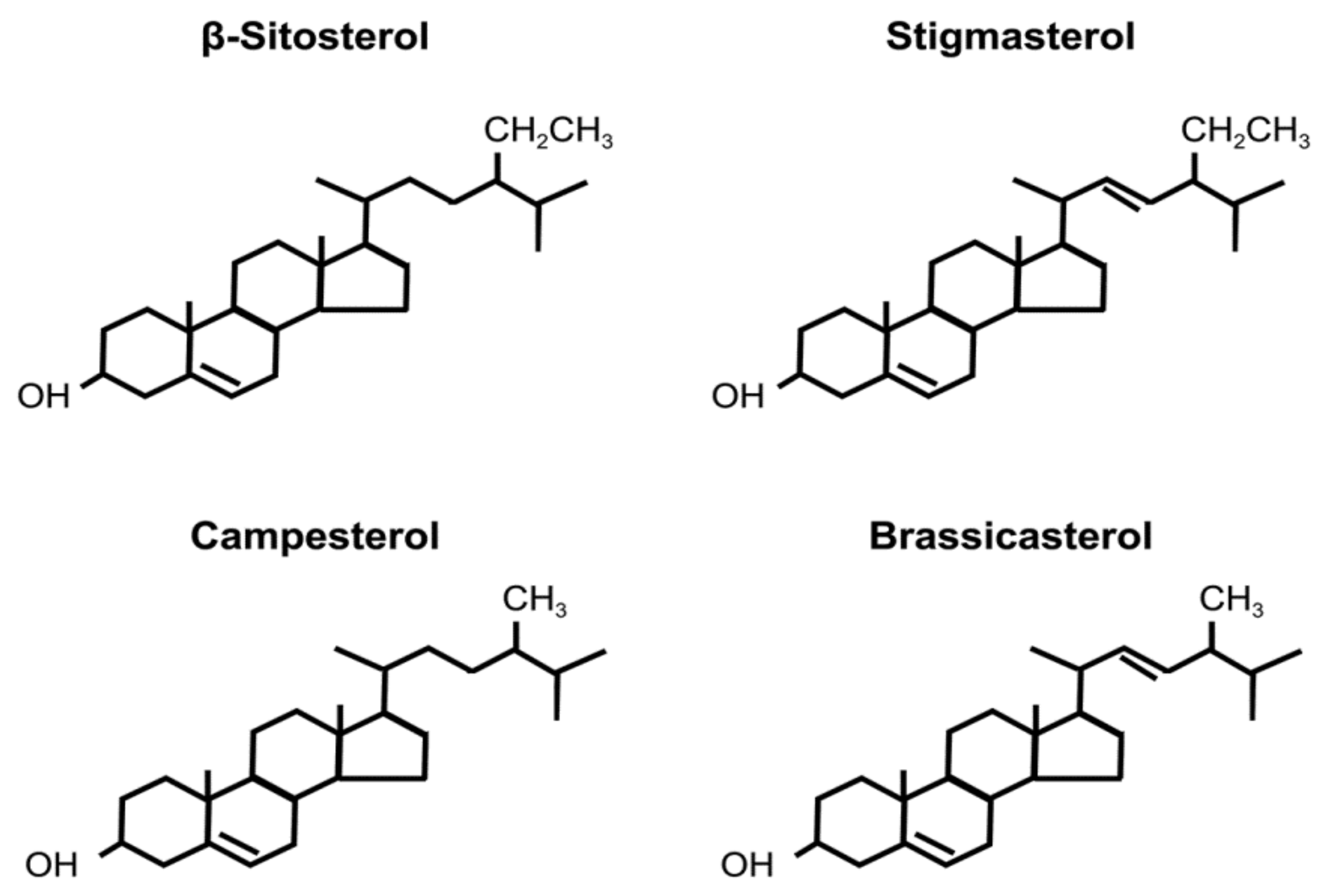
Figure 1. Structure of phytosterols.
2. Consumption of Phytosterols
Since phytosterols constitute plant cell membranes and are thus contained in most plant foods, humans routinely ingest phytosterols as constituents of plant foods, such as vegetables, grains, fruits, and vegetable oils. Phytosterols are particularly abundant in vegetable oils, cacao beans, peanuts, and broccoli [3].
Daily intake amounts of phytosterols in Japanese have been reported to be 400 mg by Nakashima et al. through a survey of university cafeterias [4] and 0.373 g by Hirai et al. [5]. The daily intake of phytosterols in children aged 6–12 years was 137 ± 65 mg according to a survey by Ishinaga et al. [6].
The following values have been described in reports from other countries. Nair et al. surveyed the suburbs of Los Angeles and reported that daily intake amounts of phytosterols in the general public and lacto-ovo vegetarian populations were 0.078 g and 0.344 g, respectively [7]. Morton et al. have reported that the phytosterol intake in British people was 0.158 g [8]. The mean daily intake of phytosterols in Dutch people was 0.289 g [9].
Furthermore, as described previously, humans worldwide have a history of daily exposure to phytosterols as constituents of plant foods since ancient times. They are thus considered to have a sufficient eating experience.
3. Safety of Phytosterols
Phytosterol safety studies focusing on acute toxicity, subacute toxicity, chronic toxicity, mutagenicity, and reproductive toxicity have shown no toxic aberrance [10][11][12][13][14][15][16]. No serious adverse reactions were found in healthy individuals, postmenopausal women, and infants with familial hypercholesterolemia after phytosterols ingestion [16][17][18][19][20].
Phytosterols reduce blood levels of some fat-soluble vitamins within the normal ranges and thus have no major effects on serum levels of fat-soluble vitamins [19][20][21][22]. In addition, phytosterols had no effects on levels of fat-soluble vitamins in the blood [17][18][23][24][25].
In healthy humans, phytosterol ingestion increases serum β-sitosterol levels only slightly. The concentration of β-sitosterol in human blood has been reported to be less than 1 mg/dL [26], which did not change after phytosterol-containing mayonnaise-type dressing materials were ingested [18][23]. The concentration was markedly lower (~1/100) than the serum β-sitosterol level in patients with β-sitosterolemia, which causes pathological symptoms [27]. Phytosterols are highly safe since they are hardly absorbed by the body [28][29][30]. Even if phytosterols are absorbed by the body from the small intestine and migrate into the blood, phytosterols are considered safe in normal humans since they are discharged from the body [31]. These data indicate that phytosterols are highly safe materials.
Moreover, phytosterols are recognized as safe. They are listed as existing food additives in Japan, and fatty acid esters of phytosterols are certified as generally recognized as safe substances in the United States.
4. Serum Cholesterol-Lowering Effect of Phytosterols
After SD rats ingested β-sitosterol, a phytosterol, along with cholesterol, the total serum cholesterol and liver cholesterol levels decreased [32]. In male CD-1 mice fed with a diet containing 1% β-sitosterol for 15 days, the liver cholesterol level decreased significantly compared with controls. No significant differences in the serum total cholesterol level were observed. In addition, although the fecal sterol excretion increased, bile acid excretion significantly decreased [33].
Golden Syrian hamsters fed with a diet containing 0.25% cholesterol and 0.01%, 0.2%, or 1% phytosterols for 45 days, and those receiving ≥0.2% phytosterols showed significant decreases in the serum and liver cholesterol levels [34].
In rats that underwent thoracic duct cannulation, administration of β-sitosterol along with cholesterol significantly reduced the amount of cholesterol recovered in the lymph [35]. When phytosterols (a mixture of β-sitosterol, campesterol, stigmasterol, and brassicasterol) were administered with cholesterol to Wistar rats, serum and liver cholesterol levels decreased, and the fecal excretion of neutral sterols increased [36].
Ooyama et al. investigated the effects of phytosterols on postprandial serum lipid levels in healthy Japanese individuals who received chicken eggs and phytosterol-containing edible oil. The results showed that phytosterol-containing edible oil suppressed the increase in postprandial RLP-C levels [37].
The data described in these reports have revealed that inhibition of cholesterol absorption from the small intestine underlies the serum total cholesterol-lowering effect of phytosterols.
5. Mechanisms Underlying Cholesterol Absorption Inhibition by Phytosterols
Complex mechanisms underpin digestion/absorption of cholesterol. Dietary cholesterol is found as free cholesterol or a cholesterol ester with a fatty acid. A cholesterol ester is hydrolyzed to free cholesterol and fatty acid in the small intestine lumen by the action of cholesterol esterase secreted by the pancreas. Hydrolyzed cholesterol forms micelles with bile acids, phospholipids, and fatty acids. The micelles enter a water layer that does not mix with the small intestine contents and is located adjacent to the microvillus membrane (unstirred water layer). In this layer, micelles release cholesterol molecules as monomers, which are taken up via simple diffusion according to a cholesterol concentration gradient across the microvillus membrane. Cholesterol molecules that are taken up by the cells are reconverted to esters with fatty acids by acyl-CoA cholesterol acyltransferase (ACAT). The resulting cholesterol esters are incorporated into chylomicrons and released into the lymph [1].
Phytosterols inhibit cholesterol absorption through a series of steps, as summarized below. Possible steps responsible for the inhibition are as follows [1]. Figure 1 shows the mechanism by which phytosterols inhibit the absorption of cholesterol.
-
Phytosterols compete against cholesterol in micelle formation, resulting in inhibition.
-
Phytosterols bind to and inhibit the cholesterol receptor essential for the cholesterol uptake across the microvillus membrane.
-
Phytosterols inhibit the intracellular esterification of cholesterol.
First, β-sitosterol, a phytosterol, has been reported to form micelles just as cholesterol does. Since only a limited amount of sterols is dissolved in micelles, the coexistence of cholesterol and β-sitosterol in the lumen of the small intestine results in a relative decrease in the cholesterol solubility in the micelle [28][35][38]. Furthermore, β-sitosterol is hardly absorbed from the small intestine [28][29] and is retained in micelles and emulsions. As a consequence, cholesterol solubility in micelles is reduced. This contributes to the decreased cholesterol absorption since cholesterol solubilization in micelles is essential for its absorption. Moreover, in rats fed with a diet containing cholesterol alone and those fed with a diet containing cholesterol and β-sitosterol, β-sitosterol decreased the cholesterol solubility in micelles, the cellular cholesterol uptake via the microvillus membrane in the small intestine [1][38], and the cholesterol recovery in the lymph by practically the same percentages [1][35]. These results demonstrate that phytosterols inhibit cholesterol absorption during cholesterol incorporation into micelles.
Second, β-sitosterol does not specifically bind to the microvillus membrane and does not competitively inhibit cholesterol absorption [1][35]. Third, β-sitosterol has no inhibitory effect on cholesterol esterification in mucosal cells of the small intestine [1][35].
Therefore, phytosterols inhibit cholesterol absorption by reducing the cholesterol solubility in micelles, lowering the serum cholesterol level.
In addition to inhibiting cholesterol absorption by phytosterols, recent findings have indicated that small amounts of phytosterols are absorbed and reach the liver. Plant sterols that reach the liver promote cholesterol’s catabolism into bile acids and excretion into bile by activating the Liver X receptor, α-CYP7A1 pathway [39]. Phytosterols reduced hepatic cholesterol level [40]. These effects reduce serum LDL cholesterol levels by reducing the hepatic cholesterol pool and the excretion of cholesterol into the blood by VLDL from the liver [41].
6. Minimum Effective Dose of Phytosterols
Pelletier et al. conducted a crossover study on 12 healthy individuals [39]. The participants ingested a butter preparation containing 740-mg/day phytosterols and a butter preparation containing no phytosterols every day for 4 weeks. When phytosterols were ingested, the total serum cholesterol and LDL-cholesterol levels were significantly lower than those when phytosterols were not ingested [42].
Sierksma et al. and Hendriks et al. tested the ingestion of 0.8- and 0.83-g/day phytosterols, respectively, and found significant decreases in the serum total cholesterol level [22][43]. Meanwhile, Miettnen et al. reported no significant difference between 0.63 and 0.65-g/day β-sitosterol and control mayonnaise [44].
Matsuoka et al. studied the minimum effective dose of phytosterols on serum cholesterol levels in Japanese participants. The results showed that the minimum effective daily dose of phytosterols for the serum cholesterol-lowering effect was likely to be 800 mg [23]. In an extensive literature survey including nearly 70 research on the phytosterol intake and serum cholesterol levels, Saito et al. concluded that the adequate intake of phytosterols was ≥800 mg [45].
This entry is adapted from the peer-reviewed paper 10.3390/foods11081141
References
- Ikeda, I. Studies on mechanism of sterols absorption. Nippon Nougeikagaku Kaishi 1991, 65, 1729–1734. (In Japanese)
- Ohno, Y.; Hara, I. New aspects on nutrition: (2) sterol. J. Jpn. Oil Chem. Soc. 1981, 30, 592–598. (In Japanese)
- Hidaka, K.; Yoshida, K.; Izaki, Y.; Toda, K. Vitamin E, cholesterol and fatty acids in foods determination of their content and Estimation of daily intake. J. Jpn. Soc. Nutr. Food Sci. 1986, 39, 308–320. (In Japanese)
- Nakajima, K.; Ikeda, I.; Fuchigami, K.; Shiroishi, Y.; Sugano, M.; Yasue, R.; Matsumoto, M. Nutritional composition of university set meal—Especially sterol and dietary fiber contents. Jap. J. Clin. Nutr. 1981, 58, 263–268. (In Japanese)
- Hirai, K.; Shimazu, C.; Takezoe, R.; Ozeki, Y. Cholesterol, phytosterol and polyunsaturated fatty acid levels in 1982 and 1957 Japanese diets. J. Nutr. Sci. Vitaminol. 1986, 32, 363–372.
- Ishinaga, M.; Mochizuki, T.; Ueda, A.; Ichi, I.; Nanatsue, M.; Oda, M.; Kishida, N. Daily intakes of fatty acids, cholesterol and plant sterols by obese and non-obese school children. J. Jpn. Soc. Nutr. Food Sci. 2001, 54, 291–296. (In Japanese)
- Nair, P.P.; Turjman, N.; Kessie, G.; Calkins, B.; Goodman, G.T.; Davidovitz, H.; Nimmagadda, G. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Dietary cholesterol, β-sitosterol, and stigmasterol. Am. J. Clin. Nutr. 1984, 40 (Suppl. S4), 927–930.
- Morton, G.M.; Lee, S.M.; Buss, D.H.; Lawrance, P. Intakes and major dietary sources of cholesterol and phytosterols in the British diet. J. Hum. Nutr. Diet. 1995, 8, 429–440.
- Normén, A.L.; Brants, H.A.M.; Voorrips, L.E.; Andersson, H.A.; van den Brandt, P.A.; Goldbohm, R.A. Plant sterol intakes and colotectal cancer risk in the Netherlands cohort study on diet and cancer. Am. J. Clin. Nutr. 2001, 74, 141–148.
- Japan Pharmaceutical Information Center. Soy unsaponifiable. Pharm. Mon. 1974, 16, 77–78. (In Japanese)
- Hepburn, P.A.; Horner, S.A.; Smith, M. Safety evaluation of phytosterol esters. Part 2. Subchronic 90-day oral toxicity study on phytosterol esters—A novel functional food. Food Chem. Toxicol. 1999, 37, 521–532.
- Shipley, R.E.; Pfeiffer, R.R.; Marsh, M.M.; Anderson, R.C. Sitosterol feeding—Chronic animal and clinical toxicology and tissue analysis. Circ. Res. 1958, 6, 373–382.
- Wolfreys, A.M.; Hepburn, P.A. Safety evaluation of phytosterol esters. Part 7. Assesment of mutagenic activity of phytosterols, phytosterol esters and the cholesterol derivative, 4-cholesten-3–one. Food Chem. Toxicol. 2002, 40, 461–470.
- Waalkens-Berendsen, D.H.; Wolterbeek, A.P.M.; Wijnands, M.V.W.; Richold, M.; Hepburn, P.A. Safety evaluation of phytosterol esters. Part 3. Two-generation reproduction study in rats with phytosterol ester—A novel functional food. Food Chem. Toxicol. 1999, 37, 683–696.
- Baker, V.A.; Hepburn, P.A.; Kennedy, S.J.; Jones, P.A.; Lea, L.J.; Sumpter, J.P.; Ashby, J. Safety evaluation of phytosterol esters. Part 1. Assessment of oestrogenicity using a combination of in vivo and in vitro assays. Food Chem. Toxicol. 1999, 37, 13–22.
- Ayesh, R.; Weststrate, J.A.; Drewitt, P.N.; Hepburn, P.A. Safety evaluation of phytosterol esters. Part 5. Feacal short-chain fatty acid and microfloracontent, feacal Bacterial enzyme activity and serum female sex hormones in healthy normolipidemic volumteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem. Toxicol. 1999, 37, 1127–1138.
- Davidson, M.H.; Maki, K.C.; Umporowicz, D.M.; Ingram, K.A.; Dicklin, M.R.; Schaefer, E.; Lane, R.W.; McNamara, J.R.; Ribaya-Mercado, J.D.; Perrone, G.; et al. Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. J. Am. Coll. Nutr. 2001, 20, 307–319.
- Matsuoka, R.; Masuda, Y.; Takeuchi, A.; Marushima, R.; Hasegawa, M.; Sakamoto, A.; Hirata, H.; Kajimoto, O.; Homma, Y. A double-blind, placebo-controlled study on the effects of mayonnaise containing free plant sterol on serum cholesterol concentration; Safety evaluation for normocholesterolemic and mildly hypercholesterolemic Japanese subjects. J. Oleo Sci. 2004, 53, 79–88. (In Japanese)
- Gylling, H.; Miettinen, T.A. Cholesterol reduction by different plant sterol mixtures and with variable fat intake. Metabolism 1999, 48, 575–580.
- Tammi, A.; Rönnemaa, T.; Gylling, H.; Rask-Nissilä, L.; Viikari, J.; Tuominen, J.; Pulkki, K.; Simell, O. Plant stanol ester margarine lowers serum total and low-density lipoprotein cholesterol concentrations of healthy children: The STRIP project. Special Turku Coronary Risk Factors Intervention Project. J. Pediatr. 2000, 136, 503–510.
- Hallikainen, M.A.; Uusitupa, M.I.J. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesteroleic subjects. Am. J. Clin. Nutr. 1999, 69, 403–410.
- Hendriks, H.F.J.; Weststrate, J.A.; van Vliet, T.; Meijer, G.W. Spreads enriched with three different levels of vagetable oil sterols and degree of cholesterol lowering in normocholesterolemic and mildly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 1999, 53, 319–327.
- Matsuoka, R.; Masuda, Y.; Takeuchi, A.; Marushima, R.; Onuki, M. Minimal effective dose of plant sterol on serum cholesterol concentration in Japanese subjects and safety evaluation of plant sterol supplemented in mayonnaise. J. Oleo Sci. 2004, 53, 17–27. (In Japanese)
- Christiansen, L.I.; Lähteenmäki, P.L.A.; Mannelin, M.R.; Seppänen-Laakso, T.E.; Hiltunen, R.V.K.; Yliruusi, J.K. Cholesterol-lowering effect of spreads enriched with microcrystallin plant sterols in hypercholesterolemic subjects. Eur. J. Nutr. 2001, 40, 66–73.
- Volpe, R.; Niittynen, L.; Korpela, R.; Sirtori, C.; Bucci, A.; Fraone, N.; Pazzucconi, F. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br. J. Nutr. 2001, 86, 233–239.
- Hidaka, H. Sitoterolemia. Nihon Rinsho 2001, 59 (Suppl. S3), 344–347. (In Japanese)
- Salen, G.; Kwiterovich, P.O.; Shefer, S.; Tint, G.S.; Horak, I.; Shore, V.; Dayal, B.; Horak, E. Increased plasma cholesterol and 5α-saturated plant sterol derivatives in subjects with sitosterolemia and xanthomatosis. J. Lipid Res. 1985, 26, 203–209.
- Ikeda, I.; Sugano, M. Inhibition of cholesterol absorption by plant sterols for mass intervention. Curr. Opin. Lipidol. 1998, 9, 527–531.
- Heinemann, T.; Axtmann, G.; von Bergmann, K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Investig. 1993, 23, 827–831.
- Ostlund, R.E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549.
- Subbiah, M.T.R.; Kuksis, A. Differences in metabolism of cholesterol and sitosterol following intravenous injection in rats. Biochim. Biophys. Acta 1973, 306, 95–105.
- Sugano, M.; Morioka, H.; Ikeda, I. A comparison of hypocholesterolemic activity of β-sitosterol and β-sitostanol in rats. J. Nutr. 1977, 107, 2011–2019.
- Uchida, K.; Mizuno, H.; Hirota, K.; Takeda, K.; Takeuchi, N.; Ishikawa, Y. Effects of spinasterol and sitosterol on plasma and liver cholesterol levels and biliary and fecal sterol and bile acid excretions in mice. Jpn. J. Pharmacol. 1983, 33, 103–112. (In Japanese)
- Ntanios, F.Y.; Jones, P.J.H. Effects of variable dietary sitostanol concentrations on plasma lipid profile and phytosterol metabolism in hamsters. Biochim. Biophys. Acta 1998, 1390, 237–244.
- Ikeda, I.; Tanaka, K.; Sugano, M.; Vahouny, G.V.; Gallo, L.L. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 1988, 29, 1573–1582.
- Ogino, Y.; Hayashi, N.; Kimura, M.; Takizawa, K.; Matsuoka, R.; Masuda, Y.; Hasegawa, M. Effect of dietary plant sterols and/or mayonnaise supplementation on lipid metabolism in rats. J. Oleo Sci. 2004, 53, 67–72. (In Japanese)
- Ooyama, K.; Seki, S.; Hidaka, I.; Yoshino, H.; Tsuji, H.; Taguchi, N.; Nakajima, S.; Kondo, K. Effects of plant sterol-containing oil on postprandial serum lipids. Jpn. J. Nutr. Diet 2001, 59, 271–276. (In Japanese)
- Ikeda, I.; Sugano, M. Some aspects of mechanism of inhibition of cholesterol absorption by β-sitosterol. Biochim. Biophys. Acta 1983, 732, 651–658.
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Molecules 2022, 27, 523.
- Matsuoka, R.; Muto, A.; Kimura, M.; Hoshina, R.; Wakamatsu, T.; Masuda, Y. Cholesterol-lowering activity of plant sterol-egg yolk lipoprotein complex in rats. J. Oleo Sci. 2008, 57, 309–314.
- Cedó, L.; Farràs, M.; Lee-Rueckert, M.; Escolà-Gil, J.C. Molecular Insights into the Mechanisms Underlying the Cholesterol- Lowering Effects of Phytosterols. Curr. Med. Chem. 2019, 26, 6704–6723.
- Pelletier, X.; Belbraouet, S.; Mirabel, D.; Mordret, F.; Perrin, J.L.; Pages, X.; Debry, G. A diet moderatery enriched in phytosterols lowers plasma cholesterol concentrations in normocholesterolemic humans. Ann. Nutr. Metab. 1995, 39, 291–295.
- Sierksma, A.; Weststrate, J.A.; Meijer, G.W. Spreads enriched with plant sterols, either esterified 4,4-dimethylsterols of free 4-desmosterols, and plasma total- and LDL-cholesterol concentrations. Br. J. Nutr. 1999, 82, 273–282.
- Vanhanen, H.T.; Miettinen, T.A. Effects of unsaturated and saturated dietary plant sterols on their serum contents. Clin. Chim. Acta 1992, 205, 97–107.
- Saito, S.; Ikeda, I.; Sugano, M. Effect of plant sterols and stanols on blood cholesterol level: Clinical evidence of minimum effective dose. J. Jpn. Soc. Nutr. Food Sci. 2002, 55, 177–189. (In Japanese)
This entry is offline, you can click here to edit this entry!
