Erythropoietin (EPO) is known as a hormone for erythropoiesis in response to anemia and hypoxia. EPO could interact with its heterodimer receptor (EPOR/βcR) to exert its anti-apoptosis, anti-inflammation and anti-oxidation effects in preventing retinal ganglion cells death through different intracellular signaling pathways.
- erythropoietin
- neuroprotection
- retinal ganglion cell
- optic neuropathy
- optic nerve protection
1. Introduction
2. EPOR: Different Isoforms with Pleiotropic Functions
2.1. The Homodimer Isoform: EPOR2
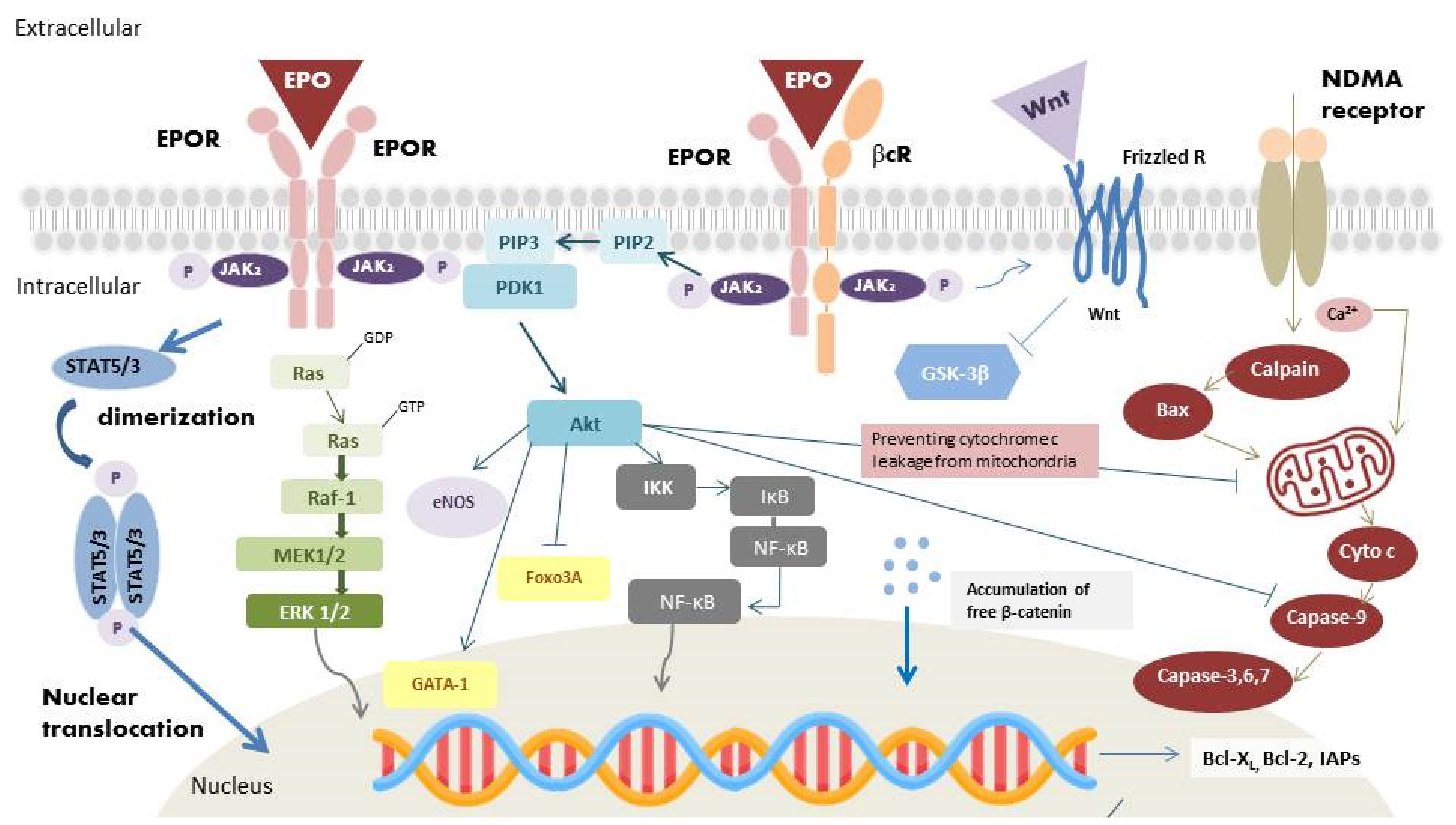
2.2. Heterodimer Isoform: EPOR/βcR
2.3. Extracellular Soluble Isoform: sEPOR
3. Effects of EPO
3.1. Angiogenic Effects
3.2. Antiapoptotic Effects
3.3. Anti-Inflammatory Effects
3.4. Antioxidant Effects
4. Current Strategy of EPO for Optic Nerve Protection and Repair
5. Advances in EPO Derivatives
Epoetin alfa (Epogen), a type of ESA medicine, has been the standard of care for patients with kidney disease and cancer-related anemia. Epoetin alfa-epbx (RetacritTM) shares the same amino acid sequence and similar carbohydrate composition as epoetin alfa (EpogenTM). In 2018, the protein was approved by the FDA, making it the first biosimilar EPO molecules approved in the USA [65]. Darbepoetin alfa (DA, Aranesp), an alternative agent of Epoetin alfa and a hyperglycosylated EPO analog, is a novel ESA with two additional N-glycosylation sites accompanied by 22 sialic acid moieties. In the attempt to extend the molecule’s half-life by three-fold longer than EPO in vivo, glycoengineering was conducted to increase the structure’s resistance to degradation. Darbepoetin alfa was approved for treating anemia resulting from renal diseases and cancer chemotherapy. The treatment protocol only requires a once-per-week visit and is accompanied by lower clinical costs [66][67]. C.E.R.A. (continuous erythropoietin receptor activator), a third-generation ESA, is an EPO (~34 kDa) integrated with methoxypolyethylene glycol (PEG, 30 kDa). Compared with other EPO derivatives, C.E.R.A. has a unique pharmacological profile with the longest half-life and slowest clearance rate. These unique pharmacological properties exist because of methoxypolyethylene glycol (PEG) integration into EPO. Notably, EPO pegylation (the process of connecting a hydrophilic polymer to EPO) significantly prolongs the duration of EPO action, and enhances proteolytic resistance in cell-free plasma [68].
Asialerythropoietin (asialoEPO) was evaluated to be a safe drug for clinical treatments. However, asialoEPO’s half-life (t1/2~1.14 min) is much shorter than that of EPO (t1/2~5.6 h). The short half-life gives asialoEPO insufficient persistence time to stimulate hematopoiesis. Based on the above concept, researchers found that chemical modification of the EPO binding sites could abolish erythropoiesis function but retain the tissue-protective effect. Carbamylated EPO (cEpo), a chemically modified derivative of EPO’s lysine residues, was found to act through the heterodimeric EPOR/βcR rather than classical EPOR2 primarily because of the modified structure of cEpo. It has confirmed that cEpo possesses neuron anti-apoptotic effects similar to EPO but instead does not induce neovascularization [69].
6. Advances in EPO Delivery
6.1. Protein-Based Ocular Delivery
6.2. Gene-Based Ocular Delivery
6.3. Surface Receptor-Targeted Ocular Delivery
6.4. Cell-Based Ocular Delivery
This entry is adapted from the peer-reviewed paper 10.3390/ijms23137143
References
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Erythropoietin in tumor angiogenesis. Exp. Cell Res. 2019, 374, 266–273.
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019, 20, 6140.
- Yasuoka, Y.; Fukuyama, T.; Izumi, Y.; Nakayama, Y.; Inoue, H.; Yanagita, K.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; et al. Erythropoietin production by the kidney and the liver in response to severe hypoxia evaluated by Western blotting with deglycosylation. Physiol. Rep. 2020, 8, e14485.
- Kimáková, P.; Solár, P.; Solárová, Z.; Komel, R.; Debeljak, N. Erythropoietin and its angiogenic activity. Int. J. Mol. Sci. 2017, 18, 1519.
- Ostrowski, D.; Heinrich, R. Alternative erythropoietin receptors in the nervous system. J. Clin. Med. 2018, 7, 24.
- Klopsch, C.; Skorska, A.; Ludwig, M.; Lemcke, H.; Maass, G.; Gaebel, R.; Beyer, M.; Lux, C.; Toelk, A.; Müller, K.; et al. Intramyocardial angiogenetic stem cells and epicardial erythropoietin save the acute ischemic heart. Dis. Models Mech. 2018, 11, dmm033282.
- Bretz, C.A.; Ramshekar, A.; Kunz, E.; Wang, H.; Hartnett, M.E. Signaling through the erythropoietin receptor affects angiogenesis in retinovascular disease. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23.
- Samson, F.P.; He, W.; Sripathi, S.R.; Patrick, A.T.; Madu, J.; Chung, H.; Frost, M.C.; Jee, D.; Gutsaeva, D.R.; Jahng, W.J. Dual switch mechanism of erythropoietin as an antiapoptotic and pro-angiogenic determinant in the retina. ACS Omega 2020, 5, 21113–21126.
- García-Ramírez, M.; Hernández, C.; Simó, R. Expression of erythropoietin and its receptor in the human retina: A comparative study of diabetic and nondiabetic subjects. Diabetes Care 2008, 31, 1189–1194.
- Constantinescu, S.N.; Ghaffari, S.; Lodish, H.F. The erythropoietin receptor: Structure, activation and intracellular signal transduction. Trends Endocrinol. Metab. TEM 1999, 10, 18–23.
- Watowich, S.S.; Hilton, D.J.; Lodish, H.F. Activation and inhibition of erythropoietin receptor function: Role of receptor dimerization. Mol. Cell. Biol. 1994, 14, 3535–3549.
- Kim, A.R.; Ulirsch, J.C.; Wilmes, S.; Unal, E.; Moraga, I.; Karakukcu, M.; Yuan, D.; Kazerounian, S.; Abdulhay, N.J.; King, D.S.; et al. Functional selectivity in cytokine signaling revealed through a pathogenic EPO mutation. Cell 2017, 168, 1053–1064.
- Tóthová, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a key protein of erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7109.
- Tóthová, Z.; Šemeláková, M.; Solárová, Z.; Tomc, J.; Debeljak, N.; Solár, P. The role of PI3K/AKT and MAPK signaling pathways in erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7682.
- Sanghera, K.P.; Mathalone, N.; Baigi, R.; Panov, E.; Wang, D.; Zhao, X.; Hsu, H.; Wang, H.; Tropepe, V.; Ward, M.; et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol. Cell. Neurosci. 2011, 47, 145–153.
- Masuda, S.; Nagao, M.; Takahata, K.; Konishi, Y.; Gallyas, F., Jr.; Tabira, T.; Sasaki, R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993, 268, 11208–11216.
- Kebschull, L.; Theilmann, L.F.C.; Mohr, A.; Uennigmann, W.; Stoeppeler, S.; Heitplatz, B.; Spiegel, H.U.; Bahde, R.; Palmes, D.M.; Becker, F. EPOR2/βcR2-independendent effects of low-dose epoetin-α in porcine liver transplantation. Biosci. Rep. 2017, 37, BSR20171007.
- Jubinsky, P.T.; Krijanovski, O.I.; Nathan, D.G.; Tavernier, J.; Sieff, C.A. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood 1997, 90, 1867–1873.
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and its derivatives: From tissue protection to immune regulation. Cell Death Dis. 2020, 11, 79.
- Colella, P.; Iodice, C.; Di Vicino, U.; Annunziata, I.; Surace, E.M.; Auricchio, A. Non-erythropoietic erythropoietin derivatives protect from light-induced and genetic photoreceptor degeneration. Hum. Mol. Genet. 2011, 20, 2251–2262.
- Vizcardo-Galindo, G.; León-Velarde, F.; Villafuerte, F.C. High-altitude hypoxia decreases plasma erythropoietin soluble receptor concentration in lowlanders. High Alt. Med. Biol. 2020, 21, 92–98.
- Dreixler, J.C.; Hagevik, S.; Hemmert, J.W.; Shaikh, A.R.; Rosenbaum, D.M.; Roth, S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology 2009, 110, 774–780.
- Khankin, E.V.; Mutter, W.P.; Tamez, H.; Yuan, H.T.; Karumanchi, S.A.; Thadhani, R. Soluble erythropoietin receptor contributes to erythropoietin resistance in end-stage renal disease. PLoS ONE 2010, 5, e9246.
- Zhang, D.; Lv, F.L.; Wang, G.H. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5071–5076.
- Ogawa, C.; Tsuchiya, K.; Tomosugi, N.; Maeda, K. A hypoxia-inducible factor stabilizer improves hematopoiesis and iron metabolism early after administration to treat anemia in hemodialysis patients. Int. J. Mol. Sci. 2020, 21, 7153.
- Socolovsky, M.; Nam, H.; Fleming, M.D.; Haase, V.H.; Brugnara, C.; Lodish, H.F. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood 2001, 98, 3261–3273.
- Tu, P.S.; Lin, E.C.; Chen, H.W.; Chen, S.W.; Lin, T.A.; Gau, J.P.; Chang, Y.I. The extracellular signal-regulated kinase 1/2 modulates the intracellular localization of DNA methyltransferase 3A to regulate erythrocytic differentiation. Am. J. Transl. Res. 2020, 12, 1016–1030.
- Dai, T.Y.; Lan, J.J.; Gao, R.L.; Zhao, Y.N.; Yu, X.L.; Liang, S.X.; Liu, W.B.; Sun, X. Panaxdiol saponins component promotes hematopoiesis by regulating GATA transcription factors of intracellular signaling pathway in mouse bone marrow. Ann. Transl. Med. 2022, 10, 38.
- Das, T.P.; Suman, S.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016, 7, e2111.
- Digicaylioglu, M.; Lipton, S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 2001, 412, 641–647.
- Shen, J.; Wu, Y.; Xu, J.Y.; Zhang, J.; Sinclair, S.H.; Yanoff, M.; Xu, G.; Li, W.; Xu, G.T. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Investig. Ophthalmol. Vis. Sci. 2010, 51, 35–46.
- Chong, Z.Z.; Kang, J.Q.; Maiese, K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2003, 23, 320–330.
- Wang, Z.Y.; Shen, L.J.; Tu, L.; Hu, D.N.; Liu, G.Y.; Zhou, Z.L.; Lin, Y.; Chen, L.H.; Qu, J. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic. Biol. Med. 2009, 46, 1032–1041.
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683.
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol. Cell. Biol. 2000, 20, 2687–2695.
- Kwak, J.; Kim, J.H.; Jang, H.N.; Jung, M.H.; Cho, H.S.; Chang, S.H.; Kim, H.J. Erythropoietin ameliorates ischemia/reperfusion-induced acute kidney injury via inflammasome suppression in mice. Int. J. Mol. Sci. 2020, 21, 3453.
- Gong, Q.; Zeng, J.; Zhang, X.; Huang, Y.; Chen, C.; Quan, J.; Ling, J. Effect of erythropoietin on angiogenic potential of dental pulp cells. Exp. Ther. Med. 2021, 22, 1079.
- Lin, X.; Ma, X.; Cui, X.; Zhang, R.; Pan, H.; Gao, W. Effects of erythropoietin on lung injury induced by cardiopulmonary bypass after cardiac surgery. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920039.
- Cui, J.; Zhang, F.; Cao, W.; Wang, Y.; Liu, J.; Liu, X.; Chen, T.; Li, L.; Tian, J.; Yu, B. Erythropoietin alleviates hyperglycaemia-associated inflammation by regulating macrophage polarization via the JAK2/STAT3 signalling pathway. Mol. Immunol. 2018, 101, 221–228.
- Elshiekh, M.; Kadkhodaee, M.; Seifi, B.; Ranjbaran, M.; Askari, H. Up-regulation of nitric oxide synthases by erythropoietin alone or in conjunction with ischemic preconditioning in ischemia reperfusion injury of rat kidneys. Gen. Physiol. Biophys. 2017, 36, 281–288.
- Cruz Navarro, J.; Pillai, S.; Ponce, L.L.; Van, M.; Goodman, J.C.; Robertson, C.S. Endothelial nitric oxide synthase mediates the cerebrovascular effects of erythropoietin in traumatic brain injury. Front. Immunol. 2014, 5, 494.
- Govindappa, P.K.; Elfar, J.C. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022, 13, 245.
- Einwächter, H.; Heiseke, A.; Schlitzer, A.; Gasteiger, G.; Adler, H.; Voehringer, D.; Manz, M.G.; Ruzsics, Z.; Dölken, L.; Koszinowski, U.H.; et al. The innate immune response to infection induces erythropoietin-dependent replenishment of the dendritic cell compartment. Front. Immunol. 2020, 11, 1627.
- Korkmaz, T.; Kahramansoy, N.; Kilicgun, A.; Firat, T. The effect of erythropoietin to pulmonary injury and mast cells secondary to acute pancreatitis. BMC Res. Notes 2014, 7, 267.
- Shokrzadeh, M.; Etebari, M.; Ghassemi-Barghi, N. An engineered non-erythropoietic erythropoietin-derived peptide, ARA290, attenuates doxorubicin induced genotoxicity and oxidative stress. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 66, 104864.
- Dang, J.Z.; Tu, Y.F.; Wang, J.; Yang, Y.J. Carbamylated erythropoietin alleviates kidney damage in diabetic rats by suppressing oxidative stress. Curr. Med. Sci. 2021, 41, 513–521.
- Salinas, M.; Wang, J.; Rosa de Sagarra, M.; Martín, D.; Rojo, A.I.; Martin-Perez, J.; Ortiz de Montellano, P.R.; Cuadrado, A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004, 578, 90–94.
- Diaz, Z.; Assaraf, M.I.; Miller, W.H., Jr.; Schipper, H.M. Astroglial cytoprotection by erythropoietin pre-conditioning: Implications for ischemic and degenerative CNS disorders. J. Neurochem. 2005, 93, 392–402.
- Thompson, A.M.; Farmer, K.; Rowe, E.M.; Hayley, S. Erythropoietin modulates striatal antioxidant signalling to reduce neurodegeneration in a toxicant model of Parkinson’s disease. Mol. Cell. Neurosci. 2020, 109, 103554.
- Pulukool, S.K.; Bhagavatham, S.K.S.; Kannan, V.; Sukumar, P.; Dandamudi, R.B.; Ghaisas, S.; Kunchala, H.; Saieesh, D.; Naik, A.A.; Pargaonkar, A.; et al. Elevated dimethylarginine, ATP, cytokines, metabolic remodeling involving tryptophan metabolism and potential microglial inflammation characterize primary open angle glaucoma. Sci. Rep. 2021, 11, 9766.
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflamm. 2019, 16, 184.
- Li, Q.; Jin, R.; Zhang, S.; Sun, X.; Wu, J. Group II metabotropic glutamate receptor agonist promotes retinal ganglion cell survival by reducing neuronal excitotoxicity in a rat chronic ocular hypertension model. Neuropharmacology 2020, 170, 108016.
- Cha, Y.W.; Kim, S.T. Serum and aqueous humor levels of brain-derived neurotrophic factor in patients with primary open-angle glaucoma and normal-tension glaucoma. Int. Ophthalmol. 2021, 41, 3869–3875.
- Mokbel, T.H.; Ghanem, A.A.; Kishk, H.; Arafa, L.F.; El-Baiomy, A.A. Erythropoietin and soluble CD44 levels in patients with primary open-angle glaucoma. Clin. Exp. Ophthalmol. 2010, 38, 560–565.
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483.
- Kawakami, M.; Sekiguchi, M.; Sato, K.; Kozaki, S.; Takahashi, M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J. Biol. Chem. 2001, 276, 39469–39475.
- Zhou, M.; Chen, S.; Wang, W.; Huang, W.; Cheng, B.; Ding, X.; Zhang, X. Levels of erythropoietin and vascular endothelial growth factor in surgery-required advanced neovascular glaucoma eyes before and after intravitreal injection of bevacizumab. Investig. Opthalmol. Vis. Sci. 2013, 54, 3874–3879.
- Sun, Y.; Zhao, H.; Shen, Y.; Guan, W. Comparison of erythropoietin, semaphorins 3A and pigment epithelium derived factor levels in serum and aqueous humor of patients with neovascular glaucoma and cataract. J. Coll. Phys. Surg. Pak. JCPSP 2019, 29, 900–901.
- Watanabe, D.; Suzuma, K.; Matsui, S.; Kurimoto, M.; Kiryu, J.; Kita, M.; Suzuma, I.; Ohashi, H.; Ojima, T.; Murakami, T.; et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005, 353, 782–792.
- Mackay, D.D. Should patients with optic neuritis be treated with steroids? Curr. Opin. Ophthalmol. 2015, 26, 439–444.
- Diem, R.; Hobom, M.; Maier, K.; Weissert, R.; Storch, M.K.; Meyer, R.; Bähr, M. Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6993–7000.
- Sättler, M.B.; Merkler, D.; Maier, K.; Stadelmann, C.; Ehrenreich, H.; Bähr, M.; Diem, R. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004, 11 (Suppl. 2), S181–S192.
- Modarres, M.; Falavarjani, K.G.; Nazari, H.; Sanjari, M.S.; Aghamohammadi, F.; Homaii, M.; Samiy, N. Intravitreal erythropoietin injection for the treatment of non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2011, 95, 992–995.
- Kashkouli, M.B.; Pakdel, F.; Sanjari, M.S.; Haghighi, A.; Nojomi, M.; Homaee, M.H.; Heirati, A. Erythropoietin: A novel treatment for traumatic optic neuropathy-a pilot study. Graefe’s Archive Clin. Exp. Ophthalmol. 2011, 249, 731–736.
- Anand, S.; Al-Mondhiry, J.; Fischer, K.; Glaspy, J. Epoetin alfa-epbx: A new entrant into a crowded market. a historical review of the role of erythropoietin stimulating agents and the development of the first epoetin biosimilar in the United States. Expert Rev. Clin. Pharmacol. 2021, 14, 1–8.
- Lee, D.E.; Son, W.; Ha, B.J.; Oh, M.S.; Yoo, O.J. The prolonged half-lives of new erythropoietin derivatives via peptide addition. Biochem. Biophys. Res. Commun. 2006, 339, 380–385.
- Powell, J.; Gurk-Turner, C. Darbepoetin alfa (Aranesp). Bayl. Univ. Med. Cent. Proc. 2002, 15, 332–335.
- Aizawa, K.; Kawasaki, R.; Tashiro, Y.; Hirata, M.; Endo, K.; Shimonaka, Y. Epoetin beta pegol, but not recombinant erythropoietin, retains its hematopoietic effect in vivo in the presence of the sialic acid-metabolizing enzyme sialidase. Int. J. Hematol. 2016, 104, 182–189.
- Liu, X.; Zhu, B.; Zou, H.; Hu, D.; Gu, Q.; Liu, K.; Xu, X. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefe’s Archive Clin. Exp. Ophthalmol. 2015, 253, 1263–1272.
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020.
- DeJulius, C.R.; Bernardo-Colón, A.; Naguib, S.; Backstrom, J.R.; Kavanaugh, T.; Gupta, M.K.; Duvall, C.L.; Rex, T.S. Microsphere antioxidant and sustained erythropoietin-R76E release functions cooperate to reduce traumatic optic neuropathy. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 762–773.
- Bond, W.S.; Hines-Beard, J.; GoldenMerry, Y.L.; Davis, M.; Farooque, A.; Sappington, R.M.; Calkins, D.J.; Rex, T.S. Virus-mediated EpoR76E therapy slows optic nerve axonopathy in experimental glaucoma. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 230–239.
- Tao, Y.; Zhu, Q.; Wang, L.; Zha, X.; Teng, D.; Xu, L. Adeno-associated virus (AAV)-mediated neuroprotective effects on the degenerative retina: The therapeutic potential of erythropoietin. Fundam. Clin. Pharmacol. 2020, 34, 131–147.
- Hines-Beard, J.; Desai, S.; Haag, R.; Esumi, N.; D’Surney, L.; Parker, S.; Richardson, C.; Rex, T.S. Identification of a therapeutic dose of continuously delivered erythropoietin in the eye using an inducible promoter system. Curr. Gene Ther. 2013, 13, 275–281.
- Bohr, S.; Patel, S.J.; Vasko, R.; Shen, K.; Iracheta-Vellve, A.; Lee, J.; Bale, S.S.; Chakraborty, N.; Brines, M.; Cerami, A.; et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J. Mol. Med. 2015, 93, 199–210.
- He, L.; Cohen, E.B.; Edwards, A.P.B.; Xavier-Ferrucio, J.; Bugge, K.; Federman, R.S.; Absher, D.; Myers, R.M.; Kragelund, B.B.; Krause, D.S.; et al. Transmembrane protein aptamer induces cooperative signaling by the EPO receptor and the cytokine receptor β-common subunit. iScience 2019, 17, 167–181.
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47.
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal stem cell therapy and alzheimer’s disease: Current status and future perspectives. J. Alzheimer’s Dis. JAD 2020, 77, 1–14.
- Luque-Campos, N.; Contreras-López, R.A.; Jose Paredes-Martínez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Front. Immunol. 2019, 10, 798.
- Jiang, Y.; Gao, H.; Yuan, H.; Xu, H.; Tian, M.; Du, G.; Xie, W. Amelioration of postoperative cognitive dysfunction in mice by mesenchymal stem cell-conditioned medium treatments is associated with reduced inflammation, oxidative stress and increased BDNF expression in brain tissues. Neurosci. Lett. 2019, 709, 134372.
- Hu, Y.; Liang, J.; Cui, H.; Wang, X.; Rong, H.; Shao, B.; Cui, H. Wharton’s jelly mesenchymal stem cells differentiate into retinal progenitor cells. Neural Regen. Res. 2013, 8, 1783–1792.
- Omoto, M.; Katikireddy, K.R.; Rezazadeh, A.; Dohlman, T.H.; Chauhan, S.K. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Investig. Opthalmol. Vis. Sci. 2014, 55, 6631–6638.
- Shirley Ding, S.L.; Kumar, S.; Ali Khan, M.S.; Ling Mok, P. Human mesenchymal stem cells expressing erythropoietin enhance survivability of retinal neurons against oxidative stress: An in vitro study. Front. Cell. Neurosci. 2018, 12, 190.
