The red king crab (RKC) is a large invasive species inhabiting bottom communities in the Barents Sea. Larval stages of RKC play an important role in determining the spread and recruitment of the population in the coastal waters. Here researchers describe morphological aspects, distribution patterns, and abunance of RKC larvae in the coastal Barents Sea.
- Paralithodes camtchaticus
- red king crab
- larvae
- invasive species
- meroplankton
- zoeae
- Barents Sea
1. Introduction
The red king crab, Paralithodes camtschaticus (Tilesius, 1815) (RKC) is one of the world’s largest crustaceans (adult males reach 12 kg in weight and 27 cm in carapace length) [1][2]. The species is native to the North Pacific and occurs from British Columbia north through the Bering Sea, and southwest to Korea [1] RKC was introduced into the Barents Sea from the Sea of Japan and the West Kamchatka waters by Russian scientists during the 1960s [3][4]. The introduction was declared to be successful, and the crab had formed a sustainable population by the mid-1990s [2][4][5]. In Russia, this new valuable fishing resource has been commercially exploited since 2004 [5][6][7][8]. In the past decade, the abundance of RKC has fluctuated significantly depending on environmental factors and fishing pressure [7][8][9], and annual landings have increased considerably [10][11]. Recently, a small-scale recreation fishery has been renewed with an annual quota of 100 t [12]. The meat of RKC is a high-quality product containing large amounts of valuable substances [13]. By-products of the crab are also rich in desirable components including chitin, chitosan, proteolytic enzymes, and fatty acids [14][15][16].
The larvae of RKC exist during the spring period and they occur in the plankton during 8–10 weeks and then settle to the bottom [4]. Larval stages are considered a crucial phase in determining the survival and stock recruitment of crabs and other crustaceans worldwide [17].
2. Larval Morphology of RCK in the Barents Sea
Four zoeal stages (zoeae I–IV) are reported for RKC [18][19]. Growth and development characteristics of each zoeal instar reared in the laboratory have been investigated by Epelbaum et al. [20] and are summarized in Table 1.
Table 1. Morphology, growth, development, and mass of zoeal stages of red king crab from the Barents Sea and North Pacific [20][21][22][23].
|
Stage |
Duration, Days |
Carapace Length, mm |
Rostrum Length, mm |
Abdomen Length, mm |
Wet Mass, mg |
Dry Mass, mg |
|
T = 7–8 °C |
Barents Sea |
|
|
|
|
|
|
Zoea I |
10 |
1.39 |
1.29 |
nd |
0.86 |
0.110 |
|
Zoea II |
10 |
1.63 |
1.52 |
nd |
1.41 |
0.165 |
|
Zoea III |
9 |
1.83 |
1.53 |
nd |
2.00 |
0.250 |
|
Zoea IV |
10 |
2.07 |
1.63 |
nd |
2.67 |
0.300 |
|
T = 8ºC |
North Pacific |
|
|
|
|
|
|
Zoea I |
12 |
1.18 |
1.45 |
2.63 |
nd |
0.045 |
|
Zoea II |
15 |
1.38 |
1.5 |
2.83 |
nd |
0.084 |
|
Zoea III |
26 |
1.45 |
1.6 |
3.25 |
nd |
0.109 |
|
Zoea IV |
33 |
1.53 |
1.3 |
3.63 |
nd |
0.191 |
Note(s): nd—no data.
Comparisons show that the zoeal stages are larger and their development is shorter in the Barents Sea than in the North Pacific (Table 1).
Zoea I has a carapace without spinules or setae on the surface (Figure 1a).
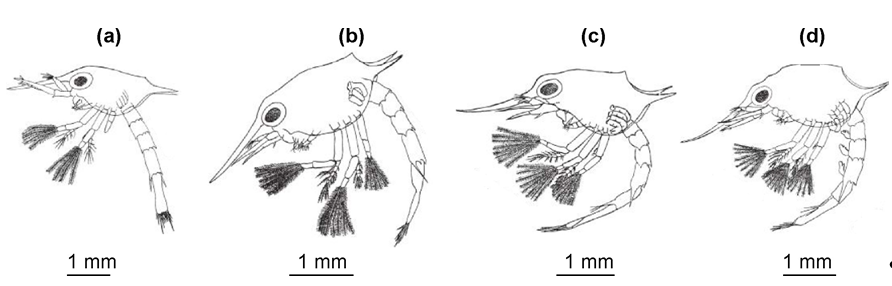
Figure 1. Common larval stages of red king crab: (a) zoea I, (b) zoea II, (c) zoea III, (d) zoea IV. Adapted from [19][22].
Rostrum elongated, slightly shorter than carapace length. There are two posterior spines. Carapace morphology is similar for all zoeal stages remaining essentially the same throughout larval development (zoeae I–IV). Antennules have a single segment and bear six olfactory setae. Antennae have a peduncle and a longer exopodite with five setae [18]. The diagnostic formula of setae on the maxillipeds is (4, 4, 0) [22]. Thoracic appendages (pereiopods) are rudimentary buds hidden beneath the carapace. The abdomen has five segments, with the last four having lateral spines (the last of which are the longest) and four small spines on the dorsal edge. The telson is fan-shaped with two symmetrical lobes separated by a medial notch, each bearing six setae and an outer spine [18]. There are two–three pairs of large yellow or green chromatophores on the carapace; arrangement of red/orange chromatophores varies [19][20].
Zoea II (Figure 1b) has a carapace, antennae, mandibles, pereiopods, abdomen, and telson proportionally higher than those of Zoea I, but otherwise unchanged [20]. The eyes are located on stalks and are movable. The Mxp setal formula is (7, 7, 6) [21]. The telson is more elongated [18].
Zoea III (Figure 1c) has a carapace, antenna, mandibles, maxillule, and telson proportionally higher than those of Zoea II, but otherwise unchanged [20]. All maxillipeds have eight setae, thus the setal formula is (8, 8, 8) [22]. The elongated telson is divided, demonstrating the rise to the sixth abdominal segment. Pairs of pleopod buds appear on abdominal segments 2 through 5, and a pair of uropod buds appears on segment 6 [18].
Zoea IV (Figure 1d) has a carapace, antenna, mandibles, maxillule, and telson proportionally higher than those of Zoea III, but otherwise unchanged [20]. The Mxp setal formula is (8, 8, 8) [22]. Thoracic appendages are visible below the carapace, and the first has a definite cheliped [18][19].
3. Abundance, Phenology, and Distribution of RCK Larvae in the Barents Sea
3.1. Horizontal Pattern
Mass hatching of RKC larvae in the Barents Sea begins in late March–early April. Females carrying developed eggs occurred in the coastal zone (40–240 m) from Varanger-fjord in the west to Maly Oleniy Island in the east (Figure 2). High densities of ovigerous females (25–100 ind. km–2) are usually recorded in the shallow waters of Medvezhya Bay, Eina Bay, Vichany Bay, Bolshaya Volokovaya Bay, Dolgaya Bay, Motovsky Bay, and Kola Bay (Figure 2). The water temperature at the bottom layer in those areas varies from 0.5 to 1.9 °C [24]. Zoeae I appear in early April [24]. The maximum density of the larvae is noted in Medvezhya Bay (52 ind. m–3) and the inner part of Motovsky Bay (18 ind. m–3) (Figure 2).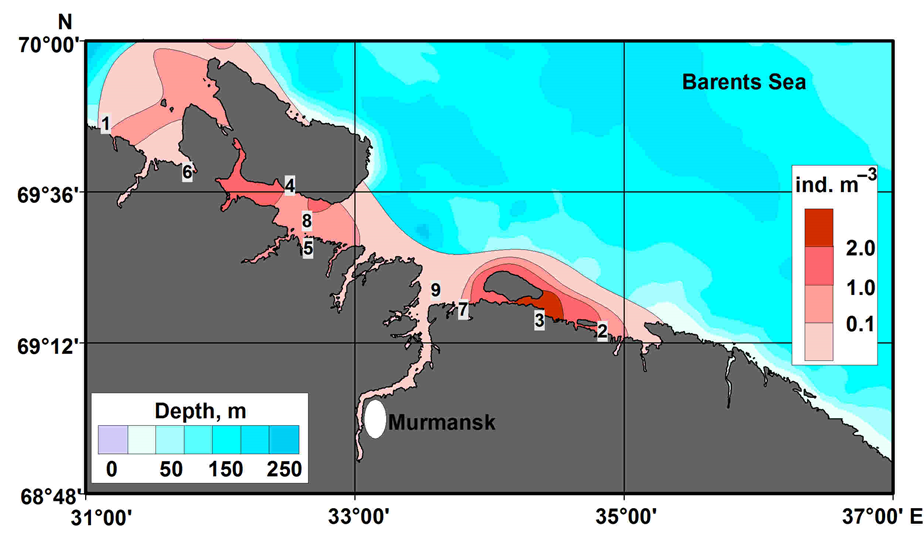
Figure 2. Distribution and abundance (individuals m–3) of red king crab larvae (zoea I) in Russian waters of the Barents Sea (spring 1996–1997) (modified from [24]). 1—Varanger-fjord, 2—Maly Oleniy Island, 3—Medvezhya Bay, 4—Eina Bay, 5—Vichany Bay, 6—Bolshaya Volokovaya Bay, 7—Dolgaya Bay, 8—Motovsky Bay, 9—Kola Bay.
First zoeae II are recorded in April but a bulk of larvae are zoeae I [24]. In May, zoeae II are found along the entire coastal waters with a maximum density (44 ind. m–3) occurring in the inner parts of the bays. The larvae are reported to prefer shallow-water sites (85–156 m) and colder waters (–0.19 °C in April and 2.15 °C in May). Zoeae III begin to occur in mid-May [24]. The larvae occur in the shallow coastal waters at 59–133 m depths from Varanger-fjord to the Seven Islands archipelago (68°50′ N, 37°12′ E) [24]. The occurrence of zoeae III in more eastern areas is probably associated with the dispersal of the larvae with the Murmansk coastal current eastward. The average abundance of zoeae III is about 0.1–0.4 ind. m–3, with maximum values in bays (1.8 ind. m–3), where the larvae exist in the plankton until the settlement due to local circulation [24]. Zoeae IV occur occasionally suggesting that their appearance would be in the late May–early June [24]. Therefore, the presence of RKC larvae in the coastal Barents Sea is proposed to be from March to mid or late June. The size of RKC larvae ranges from 2.4 to 5.8 mm, averaging 3.39 ± 0.02 mm for zoea I, 3.80 ± 0.10 mm for zoea II and 4.27 ± 0.04 mm for zoea III [24].
Table 2 summarizes data regarding the occurrence of RKC larvae in the plankton of the North Pacific region and in the Barents Sea.Table 2. Occurrence of red king crab larvae in the plankton of the Barents Sea and the North Pacific region.
|
Stage |
Region |
Period |
Reference |
|
|
Barents Sea |
|
|
|
Zoea I |
Ura Bay |
Early March–May |
|
|
|
Ura Bay |
February–May |
|
|
|
Coastal waters |
Mid–April–May |
[24] |
|
|
Coastal waters |
May |
|
|
|
Porsangerfjord |
January–April |
[33] |
|
Zoea II |
Ura Bay |
March–May |
|
|
|
Ura Bay |
February–May |
|
|
|
Coastal waters |
Mid–April–May |
[24] |
|
|
Coastal waters |
May |
|
|
|
Porsangerfjord |
April |
[33] |
|
Zoea III |
Ura Bay |
March–June |
|
|
|
Ura Bay |
April–June |
|
|
|
Coastal waters |
May |
[24] |
|
|
Coastal waters |
May |
|
|
|
Porsangerfjord |
April |
[33] |
|
Zoea IV |
Ura Bay |
April–June |
|
|
|
Ura Bay |
May–June |
|
|
|
Coastal waters |
May |
|
|
|
Open waters |
May |
[34] |
|
|
Porsangerfjord |
May–June |
[33] |
|
|
North Pacific |
|
|
|
Zoea I |
Bristol Bay |
March–July |
[35] |
|
|
Western Sakhalin waters |
March–April |
[36] |
|
|
Western Sakhalin waters |
May–June |
[22] |
|
|
Western Kamchatka waters |
March–April |
[22] |
|
|
Kamchatka waters |
April–July |
[36] |
|
|
Gulf of Alaska |
Early April–late May |
|
|
|
South–eastern Bering Sea |
Mid–April–late June |
[36] |
|
|
Aniva Bay, Sea of Japan |
April |
[39] |
|
|
The Peter Great Bay, Sea of Japan |
Late April–late May |
[22] |
|
|
Sea of Japan |
Late April–late May |
[22] |
|
Zoea II |
Gulf of Alaska |
April–June |
[38] |
|
|
Kamchatka waters |
May–July |
[36] |
|
Zoea III |
Gulf of Alaska |
Mid–April–July |
[38] |
|
|
Kamchatka waters |
June–early July |
[36] |
|
Zoea IV |
Gulf of Alaska |
Mid–April–July |
|
|
|
Tartar Strait |
Early May |
[39] |
|
|
Kamchatka waters |
June–early July |
[36] |
The time of hatching and occurrence of zoeae are similar in the Barents Sea and native areas. The appearance of larvae in the plankton was noted in populations at higher latitudes (Barents Sea and Gulf of Alaska) and in more southern Pacific regions (Sea of Japan and western coastal waters of South Sakhalin). However, there are clear differences in water temperature between the regions (<1 °C in the Barents Sea vs. 4.5–6.0 °C in the Gulf of Alaska) [37]. The period of occurrence in the plankton is also similar, while in the Pacific region, RKC larvae may be present until July in different habitats. Therefore, one can suggest that RKC larvae have a fairly wide ecological plasticity and water temperature is not a limiting factor.
3.2. Vertical Pattern
The vertical distribution of RKC larvae was studied in Medvezhya Bay (69°17’ N, 34°24’ E) [24]. Zoea I was the most abundant (56%). Zoea II accounted ca. 44% and only two zoeae III (<0.002%) were present in the plankton [24]. Zoeae I–II occurred at all water horizons during the day and formed aggregations in the surface and intermediate layers (Figure 3).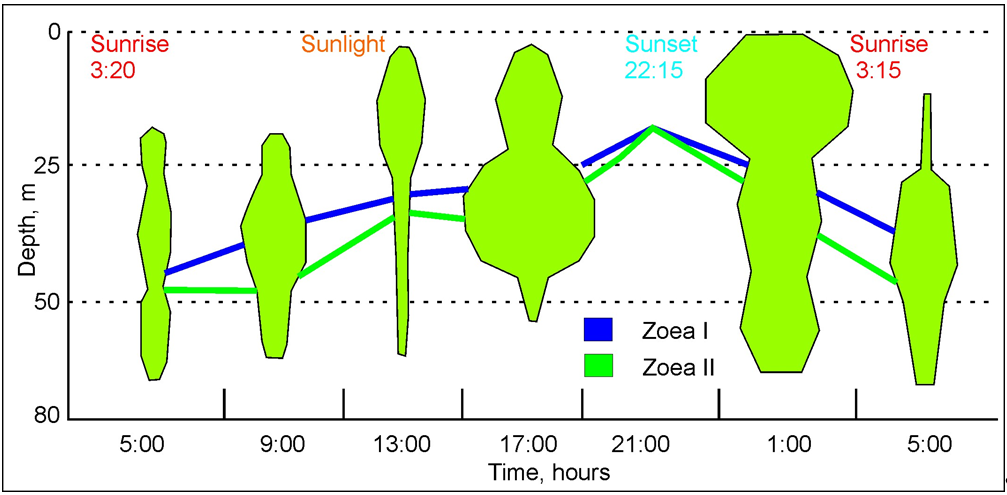
Figure 3. Vertical distribution of red king crab larvae in Russian waters of the Barents Sea (modified from [24]). The areas of the polygons are proportional to the number of RKC larvae at different depths.
Most RKC larvae occupied the intermediate layer in the morning and afternoon hours (Figure 3). The zoeae were found to move into the near-surface layer during the hours of darkness reaching the highest density at 01:00 a.m. (Figure 103 Further, there was a sinking of the larvae and they formed aggregations below 25 m by sunrise. There are no significant differences in the daily dynamics of zoea I and II, although zoea I demonstrated a smoother pattern indicating their lower mobility (Figure 3) [24]. The highest density of RKC larvae (up to 74.0 ind. m–3) was noted in the inner part at a depth of 57 m [24]. The total abundance of the zoeae ranged between 1 and 87 ind. m–3 averaging 17.5 ind. m–3 in the middle part. There was a clear decrease in the total zoeal density (14.1 ind. m–3) in the outer part whereas the open water adjacent to the bay had the lowest density [24].
4. Role of RKC Larvae in Plankton Communities in the Barents Sea
Experimental studies provided evidence that decapod larvae are omnivorous, feeding on phytoplankton and co-occurring mesozooplankton including copepod nauplii, other benthic invertebrate larvae, and conspecific and unrelated zoeae [40]. RKC larvae were also found to be plankton feeders consuming both phytoplankton and zooplankton [41]. As they pass through various stages of their development, during which they molt four times, they feed on phyto- and zooplankton in the pelagic layer for two months [18].
RKC larvae are a dominant component among decapod crustaceans existing in the plankton during the spring period. Moreover, they may amount to a considerable proportion of the total mesozooplankton in the western coastal waters. For instance, the relative density of RKC larvae can reach 70% of the total mesozooplankton biomass during the hatching period [24][32]. Their average proportion in the total mesozooplankton biomass in the coastal areas of Varanger-fjord, Motovsky Bay, and near Kola Bay varies from 1.2 to 46.4%, with maximum values being present in the shallow bays or in the inner parts of inlets [24][31][32]. There is a clear decline in the contribution of RKC zoea to the total zooplankton density towards the open sea. RKC larvae account for 0.1 ind. m–3 (<0.01% in the total mesozooplankton abundance) and 0.03 mg dry mass m–3 (0.02% in the total mesozooplankton biomass) in the southern Barents Sea [34]. In Norwegian waters, the mean proportions of RKC zoea varies from 0.02 to 0.2% of the total meroplankton in April [33][42][43].
Being a common member of meroplankton, RKC zoeae may also be ingested by macrozooplankton (e.g., medusae and ctenophores) during the spring period (Figure 4).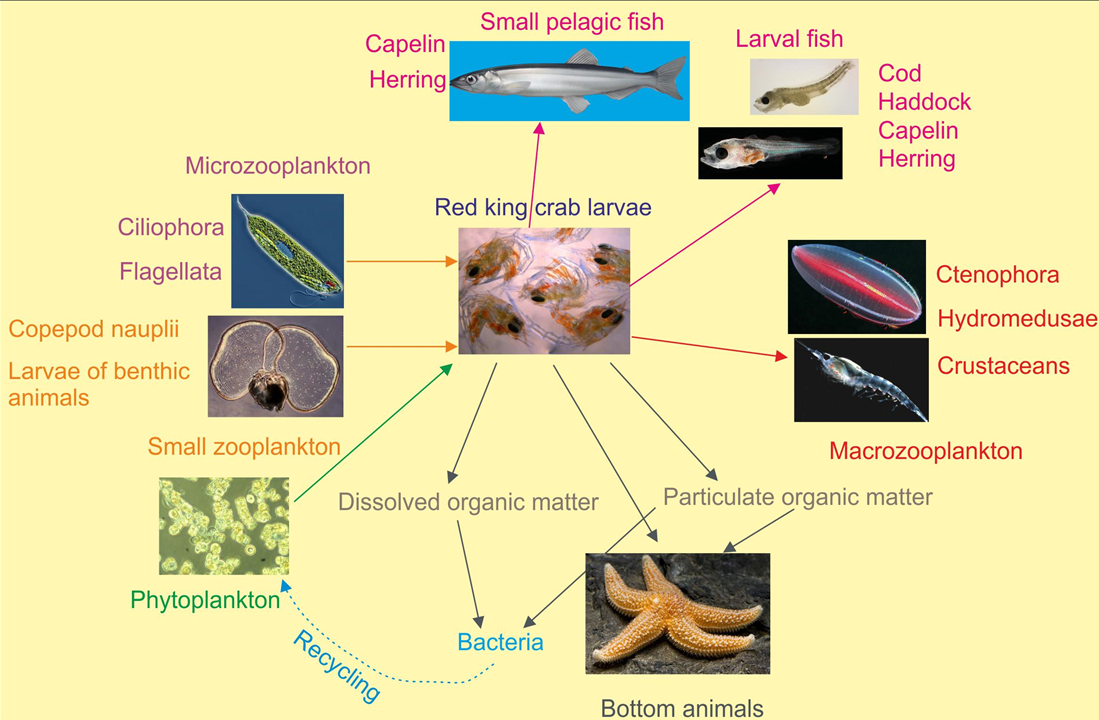
Figure 4. Trophic position of the red king crab larvae in the pelagic food web of the Barents Sea [[44]].
5. Conclusions
Larvae of Paralithodes camtschaticus RKC represent a major part of meroplankton assemblages in coastal waters during the spring period and have a measurable impact on the phyto- and zooplankton as consumers of microalgae and small pelagic animals. Mass hatching of RKC larvae occurs in April while the first zoeae can be detected in late January–February. Zoeal plankton could be detected until mid-July. Development from stage zoea I to zoea IV lasts two months. Spatial patterns of RKC larvae are mainly controlled by currents, water exchange, and advection. There is pronounced patchiness in the distribution of RKC larvae with dense aggregations being present in small bays, inlets, and inner parts of fjords. Lower abundances of RKC larvae are typical for the offshore zone. Peak density generally coincides with spring bloom. During the hatching period, the total biomass of RKC larvae can reach 70% of the total mesozooplankton biomass. Food quality and availability and environmental conditions (hydrology, circulation patterns, climatic forcing) are the main drivers determining inter-annual variability in abundance, growth, and survival rates of RKC larvae in the Barents Sea.This entry is adapted from the peer-reviewed paper 10.3390/w14152328
References
- 1. Stevens, B.G.; Lovrich, G.A. King Crabs of the World: Species and Distributions. In King crabs of the World: biology and fisheries management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, USA, 2014, pp. 1- 29.
- Dvoretsky, A.G.; Dvoretsky, V.G. 2014a. Red king crab in Russia: populations, fisheries, and symbionts. In King crabs of the World: biology and fisheries management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, USA, 2014a, pp. 501-518.
- 3. Orlov, Y.I.; Ivanov, B.G. On the introduction of the Kamchatka king crab Paralithodes camtschatica (Decapoda:Anomura: Lithodidae) into the Barents Sea. Mar. Biol. 1978, 48, 373-375.
- Kuzmin, S.A.; Gudimova, E.N. Introduction of the Kamchatka (red king) crab in the Barents Sea: peculiarities of biology, perspectives of fishery; KSC RAS Press: Apatity, Russia, 2002. (In Russian)
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331-353.
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology of red king crab in the coastal Barents Sea; SSC RAS Publishers: Rostov-on-Don, Russia, 2018. (In Russian)
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541-552.
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726.
- Dvoretsky, A.G.; Dvoretsky, V.G. 2015a. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fisheries 2015, 25, 297-322.
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917.
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: biodiversity and infestation patterns. Diversity 2022. 14, 6.
- Dvoretsky, A.G.; Dvoretsky, V.G. Renewal of the recreational red king crab fishery in Russian waters of the Barents Sea: Potential benefits and costs. Mar. Policy 2022, 136, 104916.
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition of the Barents Sea red king crab (Paralithodes camtschaticus) leg meat. J. Food Compos. Anal. 2021, 98, 103826.
- Ponomareva, T.; Timchenko, M.; Filippov, M.; Lapaev, S.; Sogorin, E. Prospects of red king crab hepatopancreas processing: fundamental and applied biochemistry. Recycling 2021, 6, 3.
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition in the hepatopancreas of the Barents Sea red king crab. Biol. Bull. 2020, 47, 332–338.
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acids in the circulatory system of an invasive king crab from the Barents Sea. J. Food Compos. Anal. 2022b. 110, 104528.
- Anger, K. Contributions of larval biology to crustacean research: a review. Invert. Repr. Dev. 2006, 49, 175-205.
- Stevens, B.G. Development and Biology of King Crab Larvae. In King crabs of the World: biology and fisheries management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, USA, 2014, pp. 233-259.
- 19. Marukawa, H. Biology and fishery research on Japanese king crab Paralithodes camtschatica. J. Imper. Fish. Exper. Sta. Tokyo, 1933, 1-152.
- Epelbaum, A.B.; Borisov, R.R.; Kovatcheva, N.P. Early development of the red king crab Paralithodes camtschaticus from the Barents Sea reared under laboratory conditions: Morphology and behaviour. J. Mar. Biol. Assoc. UK 2006, 86, 317-333.
- Sato, S.; Tanaka S. Study on the larval stage of Paralithodes camtschatica (Tilesius) I. About morphological research. Bull. Hokkaido Reg. Fish. Res. Lab. 1949, 1, 7-24.
- 22. Sato, S. Studies on larval development and fishery biology of king crab, Paralithodes camtschatica (Tilesius). Bull. Hokkaido Reg. Fish. Res. Lab. 1958, 17, 1-102.
- Nakanishi, T. Rearing condition of eggs, larvae and post-larvae of king crab. Bull Japan Sea Reg Fish Lab. 1987, 37, 57-161.
- Bakanev, S.V. Larvae of red king crab in the coastal areas and large bays of Murman. In The red king crab in the Barents Sea. Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003b. pp. 122-133. (In Russian)
- Matyushkin, V.B.; Ushakova, M.F. Features of the larval cycle of red king crab (Paralithodes camtschaticus) and hermit crab (Pagurus pubescens) in the fjord waters of Western Murman. In Bioresources and aquaculture in the coastal areas of the Barents and White Seas. PINRO Press: Murmansk, Russia, 2002, pp. 125-136. (In Russian)
- Matyushkin, V.B.; Ushakova, M.F. Larvae of red king crab in the fjords of Western Murman In The red king crab in the Barents Sea. Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003b. pp. 133-140. (In Russian)
- Ushakova, M.V. Distribution and abundance of larvae of some common crustacean species of in the coastal waters of the Western Murman. In Management of the coastal zone in the northern seas. St. Petersburg State Univercity: St. Petersburg, Russia, 1999. pp. 184-188. (In Russian)
- Shamray, T.V. Changes in the abundance and terms of presence in the plankton of the red king crab larvae within the Ura Bay (West Murman) in 2011-2016. Vestn. MGTU 2017, 20, 493-502. (In Russian)
- Shamray, T.V. Distribution of pelagic larvae of some representatives of the Decapoda order in the coastal waters of Western Murman. In Biological resources of fishing off the coast of Murmansk. Sokolov, V.M., Ed.; PINRO Press: Murmansk, Russia, 2013. pp. 129-140. (In Russian)
- Shamray, T.V., Matushkin V.B. Larvae of the red king crab in the coastal waters of Western Murman. In The red king crab in the Barents Sea. Bizikov, V.A., Stesko, A.V., Alexeev, D.O., Buyanovsky, A.I., Dolgov, A.V., Novikov, M.A., Pereladov, M.V., Sentyabov, E.V., Sokolov, K.M., Eds.; VNIRO Publishing: Moscow, Russia, 2021, pp. 223-239. (In Russian)
- Dvoretskii, V.G. Distribution of euphausiid and decapod larvae in the spring plankton of the southern Barents Sea. Biol. Bull. 2011, 38, 393–399
- Dvoretsky, V.G.; Dvoretsky, A.G. Ecology of zooplankton communities in the Barents Sea and adjacent waters. Renome: St. Petersburg Russia. 2015. (In Russian)
- Michelsen, H.K.; Nilssen, E.M.; Pedersen, T.; Svensen, C. Temporal and spatial dynamics of the invasive red king crab and native brachyuran and anomuran larvae in Norwegian waters. Aquat. Biol. 2020, 29, 1-16.
- Dvoretsky, V.G.; Dvoretsky, A.G. Zooplankton productivity in the coastal area of the southern Barents Sea in spring. Mar. Biol. J. 2020, 5, 3-14.
- Otto R.S.; Macintosh R.A.; Cummiskey P.A. Fecundity and other reproductive parameters of female red king crab (Paralithodes camtschaticus) in Bristol Bay and Norton Sound, Alaska. In Proc. of the Intern. Symposium on King and Tanner Crabs. Univ. Alaska Sea Grant Rep., 1989, pp. 65-90.
- Makarov, R.R. Larvae of shrimps, hermit crabs and crabs of the Western Kamchatka shelf and their distribution. Nauka Publishing: Moscow, Russia, 1966. (In Russian)
- Paul A.J., Paul J.M. Coyle K.O. Energy sources for first-feeding zoeae of king crab Paralithodes camtschatica (Tilesius). J. Exp. Mar. Biol. Ecol. 1989, 130, 55-69.
- Paul, A. J., Paul J. M., 1990. Growth of stage I king crab larvae of Paralithodes camtschatica (Tilesius) (Decapoda:Lithodidae) in natural communities. J. Crust. Biol. 10, 175-183.
- Klitin, A.K.; Samatov A.D. Role of larvae dispersal in population dynamics of the red king crab in Tatar Strait. In Fisheries investigations of the World's Ocean. Dalrybvtuz Press: Valdivostok, Russia, 1999, pp. 140-142. (In Russian)
- Harms, J.; Seeger, B. Larval development and survival in seven decapod species (Crustacea) in relation to laboratory diet. J. Exp. Mar. Biol. Ecol. 1989, 133, 129-139.
- Bright, D.B. Life histories of the king crab, Paralithodes camtschatica, and the Tanner crab, Chionoecetes bairdi, in Cook Inlet, Alaska. Ph.D. thesis, Univ. South. Calif.: Los Angeles. 1967.
- Michelsen, H.K.; Svensen, C.; Reigstad, M.; Nilssen, E.M.; Pedersen, T. Seasonal dynamics of meroplankton in a high-latitude fjord. J. Mar. Syst. 2017, 168, 17-30.
- Michelsen, H.K.; Nilssen, E.M.; Pedersen, T.; Reigstad, M.; Svensen, C. Spatial patterns of spring meroplankton along environmental gradients in a sub-Arctic fjord. Aquat, Biol, 2017, 26, 185-197.
- Dvoretsky, V.G., Dvoretsky, A.G. Ecology and distribution of red king crab larvae in the Barents Sea: a review. Water 2022. 14, 2328.
